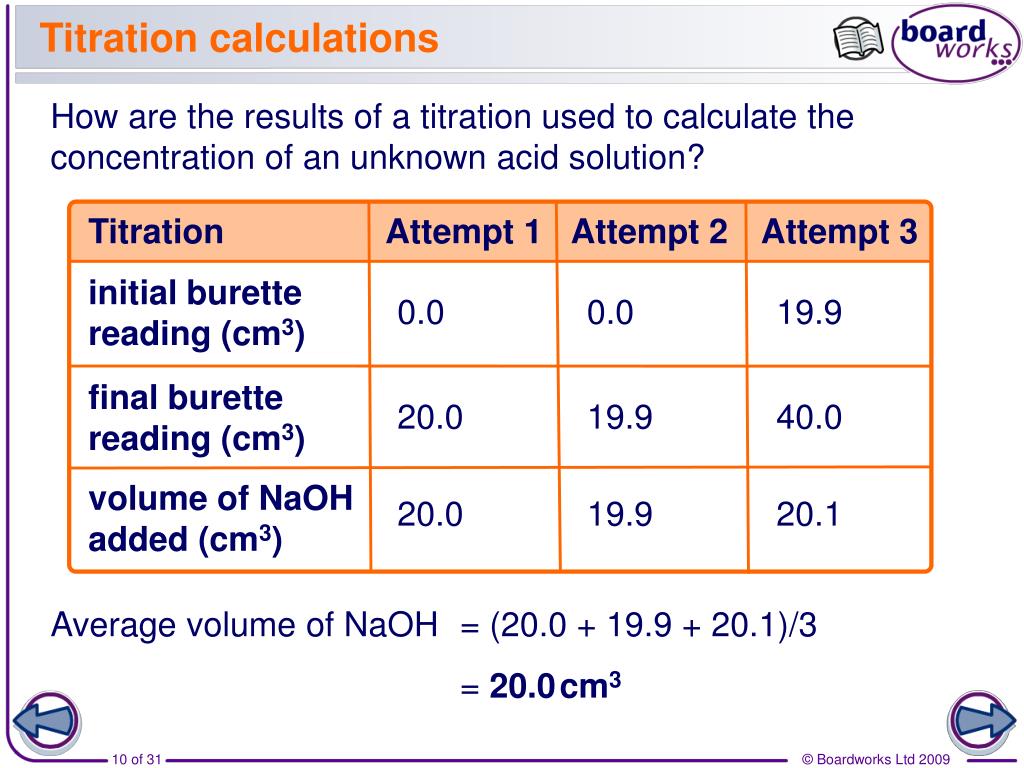
How To Do A Titration And Calculate The Concentration . Let's assume you are titrating a strong acid (10 ml unknown. Concordant titres should be used when. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. How do you calculate concentration from titration? A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Data collected during a titration allows chemists to determine a solution’s unknown concentration. They can also be used to calculate the ph after a given point during a titration M av a = m bv b. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The amount of added titrant is determined from its concentration and volume: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. First determine the moles of \(\ce{naoh}\) in the reaction. Titration calculations are used to find the concentration of unknown solutions; The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator.
from www.slideserve.com
The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. They can also be used to calculate the ph after a given point during a titration How do you calculate concentration from titration? Concordant titres should be used when. Let's assume you are titrating a strong acid (10 ml unknown. Titration calculations are used to find the concentration of unknown solutions; M av a = m bv b. First determine the moles of \(\ce{naoh}\) in the reaction.
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214
How To Do A Titration And Calculate The Concentration Data collected during a titration allows chemists to determine a solution’s unknown concentration. Let's assume you are titrating a strong acid (10 ml unknown. They can also be used to calculate the ph after a given point during a titration Titration calculations are used to find the concentration of unknown solutions; N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. How do you calculate concentration from titration? Concordant titres should be used when. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: M av a = m bv b. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Data collected during a titration allows chemists to determine a solution’s unknown concentration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co How To Do A Titration And Calculate The Concentration They can also be used to calculate the ph after a given point during a titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Let's assume you are titrating a strong acid (10 ml unknown. First. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
Titration Calculations YouTube How To Do A Titration And Calculate The Concentration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Let's. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How To Do A Titration And Calculate The Concentration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Titration calculations are used to find the concentration of unknown solutions; First determine the moles of \(\ce{naoh}\) in the reaction. They can also be used to calculate the ph after a given point during a titration A titration is a laboratory technique used to precisely. How To Do A Titration And Calculate The Concentration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Do A Titration And Calculate The Concentration The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. M av a = m bv b. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable. How To Do A Titration And Calculate The Concentration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Foundation How To Do A Titration And Calculate The Concentration First determine the moles of \(\ce{naoh}\) in the reaction. Titration calculations are used to find the concentration of unknown solutions; Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. They can also be used to calculate the ph after a given point during a titration Concordant titres should be used when. The amount of. How To Do A Titration And Calculate The Concentration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID4401864 How To Do A Titration And Calculate The Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Let's assume you are titrating a strong acid (10 ml unknown. Data collected during a titration allows chemists to determine a solution’s unknown concentration. They. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Questions YouTube How To Do A Titration And Calculate The Concentration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Let's assume you are titrating a strong acid (10 ml unknown. The volumes of acids and alkali solutions that react with each other can be measured by titration. How To Do A Titration And Calculate The Concentration.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Foundation How To Do A Titration And Calculate The Concentration How do you calculate concentration from titration? Concordant titres should be used when. Titration calculations are used to find the concentration of unknown solutions; N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved.. How To Do A Titration And Calculate The Concentration.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Foundation How To Do A Titration And Calculate The Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Concordant titres should be used when. The amount of added titrant is determined from its concentration and volume: Titration is a method to determine the unknown concentration. How To Do A Titration And Calculate The Concentration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Do A Titration And Calculate The Concentration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. Concordant titres should be used when. Let's assume you are titrating a strong acid (10 ml unknown. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Data collected. How To Do A Titration And Calculate The Concentration.
From mavink.com
Titration Reaction How To Do A Titration And Calculate The Concentration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. M av a = m bv b. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. They can also be used to calculate the ph after a. How To Do A Titration And Calculate The Concentration.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co How To Do A Titration And Calculate The Concentration How do you calculate concentration from titration? Let's assume you are titrating a strong acid (10 ml unknown. The amount of added titrant is determined from its concentration and volume: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. N (mol) = c (mol /l) * v (l) and. How To Do A Titration And Calculate The Concentration.
From www.aplustopper.com
How does titration determine concentration? A Plus Topper How To Do A Titration And Calculate The Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. They can also be used to calculate the ph after a given point during a titration M av a = m bv b. From the. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry YouTube How To Do A Titration And Calculate The Concentration How do you calculate concentration from titration? First determine the moles of \(\ce{naoh}\) in the reaction. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted.. How To Do A Titration And Calculate The Concentration.
From theedge.com.hk
Chemistry How To Titration The Edge How To Do A Titration And Calculate The Concentration Concordant titres should be used when. The amount of added titrant is determined from its concentration and volume: M av a = m bv b. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. They can also be used to calculate the ph after a given point during. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
How to do a titration and calculate the concentration YouTube How To Do A Titration And Calculate The Concentration How do you calculate concentration from titration? First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: Let's assume you are titrating a strong acid (10 ml unknown. Concordant titres should be used when. Titration calculations are used to find the concentration of unknown solutions; Data collected during a. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution requires 83 6 mL of 0 12 How To Do A Titration And Calculate The Concentration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Titration calculations are used to find the concentration of unknown solutions; First determine. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
CHEM 101 Titration of Unknown Triprotic Acid YouTube How To Do A Titration And Calculate The Concentration Titration calculations are used to find the concentration of unknown solutions; A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. They can also be used to calculate the ph after a given point during a titration N (mol) = c (mol /l) * v (l) and the amount of. How To Do A Titration And Calculate The Concentration.
From es.wikihow.com
Cómo hacer una titulación (con imágenes) wikiHow How To Do A Titration And Calculate The Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Let's assume you are titrating a strong acid (10 ml unknown. They can also be used to calculate the ph after a given point during a titration First determine the moles of \(\ce{naoh}\) in the reaction. The volumes of acids. How To Do A Titration And Calculate The Concentration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Do A Titration And Calculate The Concentration Let's assume you are titrating a strong acid (10 ml unknown. M av a = m bv b. The amount of added titrant is determined from its concentration and volume: Titration calculations are used to find the concentration of unknown solutions; Concordant titres should be used when. First determine the moles of \(\ce{naoh}\) in the reaction. How do you calculate. How To Do A Titration And Calculate The Concentration.
From www.wikihow.com
5 Easy Ways to Calculate the Concentration of a Solution How To Do A Titration And Calculate The Concentration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. Titration calculations are used to find the concentration of unknown solutions; Concordant titres should be used when. Let's assume you are titrating a strong acid (10 ml unknown. Data collected during a titration allows chemists to determine a solution’s unknown. How To Do A Titration And Calculate The Concentration.
From www.science-revision.co.uk
Titrations How To Do A Titration And Calculate The Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Concordant titres should be used when. The amount. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry How To Do A Titration And Calculate The Concentration Let's assume you are titrating a strong acid (10 ml unknown. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. How do you calculate concentration from titration? Titration calculations are used to find the concentration of unknown solutions; Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Titration. How To Do A Titration And Calculate The Concentration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Do A Titration And Calculate The Concentration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. M av a = m bv b. Let's assume you are titrating a strong acid (10 ml unknown. The amount of added titrant is determined from its concentration and volume: Titration is a method to determine the unknown concentration. How To Do A Titration And Calculate The Concentration.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d) TRIPLE Teaching Resources How To Do A Titration And Calculate The Concentration First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Concordant titres should be used when. Titration is a method to determine the unknown concentration of a specific substance (analyte). How To Do A Titration And Calculate The Concentration.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic How To Do A Titration And Calculate The Concentration The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. How do you calculate concentration from titration? Titration is a method to determine the unknown concentration of a. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Do A Titration And Calculate The Concentration The amount of added titrant is determined from its concentration and volume: Concordant titres should be used when. How do you calculate concentration from titration? They can also be used to calculate the ph after a given point during a titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Data collected during a titration allows chemists to. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube How To Do A Titration And Calculate The Concentration The amount of added titrant is determined from its concentration and volume: How do you calculate concentration from titration? A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Concordant titres should be used when. Let's assume you are titrating a strong acid (10 ml unknown. M av a =. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate and molarity YouTube How To Do A Titration And Calculate The Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. How do you calculate concentration from titration? They can also be used to calculate the ph after a given point during a titration Data collected during a titration allows chemists to determine a solution’s unknown concentration. The amount of added. How To Do A Titration And Calculate The Concentration.
From www.tutordale.com
How To Calculate Concentration In Chemistry How To Do A Titration And Calculate The Concentration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. M av a = m bv b. Concordant titres should be used when. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. From the mole ratio, calculate the. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
[Example] How to Find the Concentration of a Solution. YouTube How To Do A Titration And Calculate The Concentration Concordant titres should be used when. How do you calculate concentration from titration? Titration calculations are used to find the concentration of unknown solutions; M av a = m bv b. They can also be used to calculate the ph after a given point during a titration From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution YouTube How To Do A Titration And Calculate The Concentration The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. M av a = m bv b. How do you calculate concentration from titration? N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio,. How To Do A Titration And Calculate The Concentration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download ID3199214 How To Do A Titration And Calculate The Concentration Titration calculations are used to find the concentration of unknown solutions; The amount of added titrant is determined from its concentration and volume: First determine the moles of \(\ce{naoh}\) in the reaction. Concordant titres should be used when. The volumes of acids and alkali solutions that react with each other can be measured by titration using a suitable indicator. Data. How To Do A Titration And Calculate The Concentration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple How To Do A Titration And Calculate The Concentration Concordant titres should be used when. How do you calculate concentration from titration? Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. First determine the moles of \(\ce{naoh}\) in the reaction. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Data collected. How To Do A Titration And Calculate The Concentration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Khan Academy YouTube How To Do A Titration And Calculate The Concentration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. First determine the moles of \(\ce{naoh}\) in the reaction. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Let's assume you are titrating a strong acid (10 ml unknown. Data collected during a. How To Do A Titration And Calculate The Concentration.