Cleaning Chemical Residue Testing . To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. Unexpected swab results led to an investigation, which. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. a current cleaning validation study evaluated both swab testing and vrl. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. you may benefit from a chemical that can be used as a cleaner and sanitizer. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and.
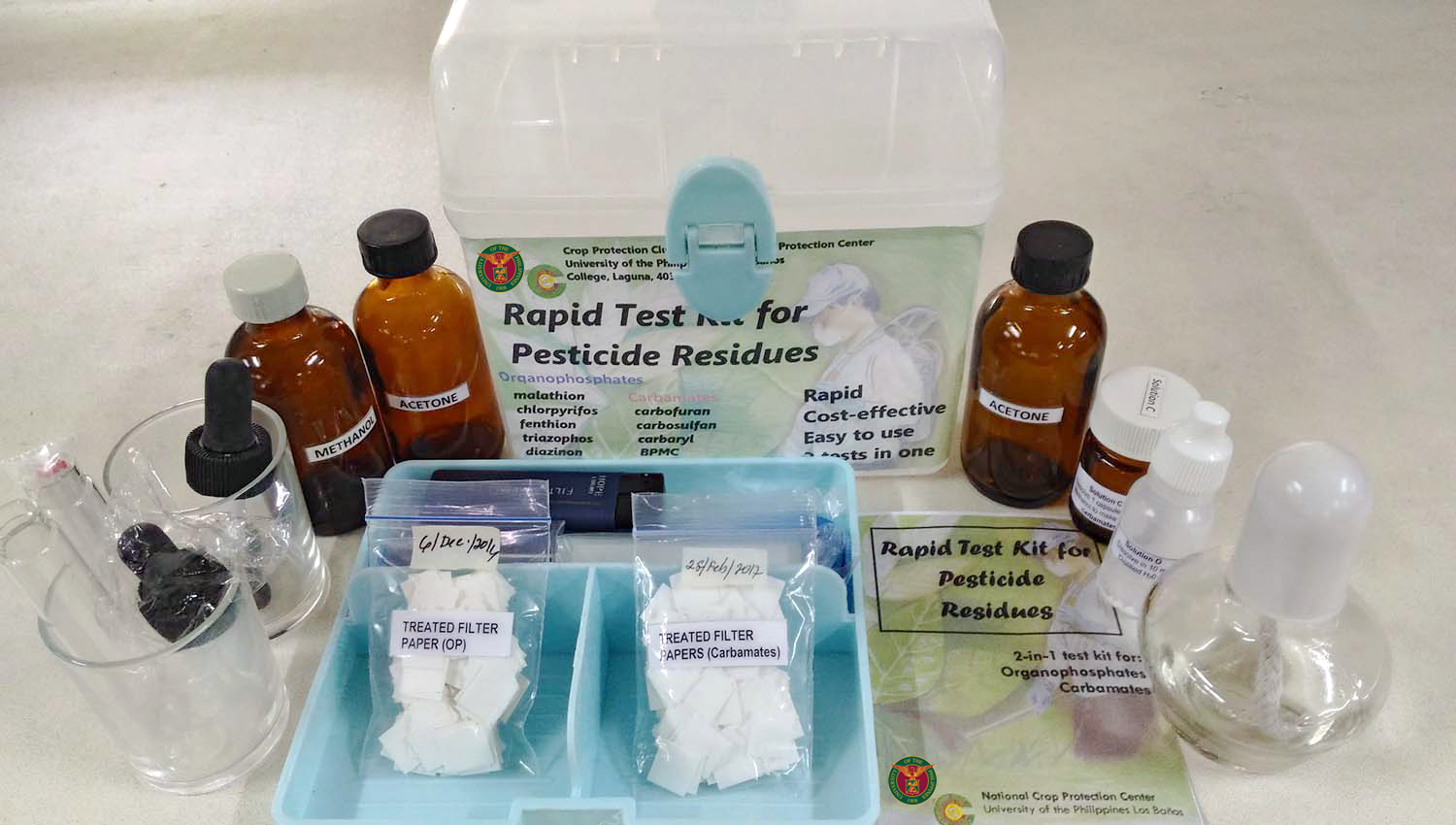
from agora.uplb.edu.ph
cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. a current cleaning validation study evaluated both swab testing and vrl. Unexpected swab results led to an investigation, which. you may benefit from a chemical that can be used as a cleaner and sanitizer. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant.
Rapid Test Kits for Pesticide Residue UPLB AGORA
Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. you may benefit from a chemical that can be used as a cleaner and sanitizer. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. Unexpected swab results led to an investigation, which. a current cleaning validation study evaluated both swab testing and vrl.
From www.youtube.com
Drug Residue Testing Kit Dipstick Technology Protocol YouTube Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. you may benefit from a chemical that can be used as a cleaner and sanitizer. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. cleanliness testing allows manufacturers to identify foreign residue or particles present in components. Cleaning Chemical Residue Testing.
From www.youtube.com
Pesticide Residue Analysis Sample Preparation Extraction and Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. you may benefit from a chemical that can. Cleaning Chemical Residue Testing.
From www.thermofisher.com
Pesticide Residues Testing Information Thermo Fisher Scientific JP Cleaning Chemical Residue Testing you may benefit from a chemical that can be used as a cleaner and sanitizer. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. . Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. To give a comprehensive view of. Cleaning Chemical Residue Testing.
From www.thermofisher.com
Pesticide Residues Testing Information Thermo Fisher Scientific IN Cleaning Chemical Residue Testing To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on. Cleaning Chemical Residue Testing.
From www.vecteezy.com
Scientist check chemical fruit residues in laboratory. Control experts Cleaning Chemical Residue Testing cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. you may benefit from a chemical that can be used as a cleaner and sanitizer. a current cleaning validation study. Cleaning Chemical Residue Testing.
From www.novumlifesciences.com.au
Chemical Residue Novum Lifesciences Cleaning Chemical Residue Testing Unexpected swab results led to an investigation, which. a current cleaning validation study evaluated both swab testing and vrl. you may benefit from a chemical that can be used as a cleaner and sanitizer. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. cleanliness testing allows manufacturers to. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the.. Cleaning Chemical Residue Testing.
From glenwood.ph
Chemical Residue Testing Cleaning Chemical Residue Testing To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. a current cleaning validation study evaluated both swab testing and vrl. you may benefit from a chemical that can. Cleaning Chemical Residue Testing.
From glenwood.ph
Chemical Residue Testing Cleaning Chemical Residue Testing you may benefit from a chemical that can be used as a cleaner and sanitizer. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. a current cleaning validation study evaluated both swab testing and vrl. cleaning validation procedures are carried out in order to assure that residues of. Cleaning Chemical Residue Testing.
From glenwood.ph
Chemical Residue Testing Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. a current cleaning validation study evaluated both swab testing and vrl. you may benefit from a chemical that can be used as a cleaner and sanitizer. our chemical residue and contaminant analysis and testing provides detection to trace residue. Cleaning Chemical Residue Testing.
From blog.thepipingmart.com
Procedure of Chemical Cleaning ThePipingMart Blog Cleaning Chemical Residue Testing To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. Unexpected swab results led to an investigation, which. cleaning validation procedures are carried. Cleaning Chemical Residue Testing.
From methlabs.com.au
Meth Lab Decontamination Meth Lab Cleanup & Drug Residue Testing Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. you may benefit from a chemical that can be used as a cleaner and sanitizer. test methods are available to quantify the amount of residuals. Cleaning Chemical Residue Testing.
From melbournemethtesting.com.au
How to clean meth residue in a house? Melbourne Meth Testing Solutions Cleaning Chemical Residue Testing To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. our chemical residue. Cleaning Chemical Residue Testing.
From www.agri-instrument.com
Pesticide Residue Tester Testing Analysis Cleaning Chemical Residue Testing Unexpected swab results led to an investigation, which. a current cleaning validation study evaluated both swab testing and vrl. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. our chemical residue and contaminant analysis and testing provides detection to trace residue levels. Cleaning Chemical Residue Testing.
From alibaba.com
Pesticide Residue Detection Kit In Food Buy Food Testing Kits Product Cleaning Chemical Residue Testing you may benefit from a chemical that can be used as a cleaner and sanitizer. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. To give. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. Unexpected swab results led to an investigation, which. a current cleaning validation study evaluated both swab testing and vrl. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the. Cleaning Chemical Residue Testing.
From www.innovatechlabs.com
Why Residual Solvent Testing is a Must Cleaning Chemical Residue Testing you may benefit from a chemical that can be used as a cleaner and sanitizer. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. test methods are available to. Cleaning Chemical Residue Testing.
From glenwood.ph
Chemical Residue Testing Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. you may benefit from a chemical that can be used as a cleaner and sanitizer. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. a current cleaning validation. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. Unexpected swab results led to an investigation, which. a current cleaning validation study evaluated both swab testing and vrl. you may benefit. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. cleanliness testing allows manufacturers. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. Unexpected swab results led to an investigation, which. you may benefit from a chemical that can be used as a cleaner and sanitizer. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and. Cleaning Chemical Residue Testing.
From glenwood.ph
Chemical Residue Testing Cleaning Chemical Residue Testing cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. a current cleaning validation study evaluated both swab testing and vrl. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. To give a comprehensive view of. Cleaning Chemical Residue Testing.
From shop.mhp-verlag.de
Validation of the cleaning method for da Vinci XI robotic instruments Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. you may benefit from a chemical that can be used as a cleaner and sanitizer. Unexpected swab results led to an investigation, which. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. a current cleaning validation study evaluated both swab testing and vrl. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. cleanliness testing allows manufacturers. Cleaning Chemical Residue Testing.
From www.dreamstime.com
Pesticide residue testing stock photo. Image of research 9043492 Cleaning Chemical Residue Testing To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. you may benefit from a chemical that can be used as a cleaner and sanitizer. cleanliness testing allows manufacturers. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. our. Cleaning Chemical Residue Testing.
From www.youtube.com
Residues Testing YouTube Cleaning Chemical Residue Testing To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. you may benefit. Cleaning Chemical Residue Testing.
From www.youtube.com
Cannalysis Chemical Residue Testing YouTube Cleaning Chemical Residue Testing Unexpected swab results led to an investigation, which. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. you may benefit from a chemical that can be used as a. Cleaning Chemical Residue Testing.
From glenwood.ph
Chemical Residue Testing Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to. Cleaning Chemical Residue Testing.
From lonestaralpha.com
Pesticide Residues Testing Lonestar Alpha Lab Cleaning Chemical Residue Testing cleaning validation procedures are carried out in order to assure that residues of cleaning agents are within. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative. Cleaning Chemical Residue Testing.
From agora.uplb.edu.ph
Rapid Test Kits for Pesticide Residue UPLB AGORA Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. Unexpected swab results led to an. Cleaning Chemical Residue Testing.
From www.carolina.com
Gunshot and Explosive Residue Testing Kit Carolina Biological Supply Cleaning Chemical Residue Testing To give a comprehensive view of cleanliness, you can also include a cytotoxicity assessment and tests to examine the microbiological contamination. test methods are available to quantify the amount of residuals (i.e., oils, lubricants, polishing compounds, detergents, and passivation residuals) on the device or implant. our chemical residue and contaminant analysis and testing provides detection to trace residue. Cleaning Chemical Residue Testing.
From www.youtube.com
BioeasyPesticide Residues Rapid Test Kit YouTube Cleaning Chemical Residue Testing a current cleaning validation study evaluated both swab testing and vrl. our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. cleanliness testing allows manufacturers to identify foreign residue or particles present in components and identify the. cleaning validation procedures are carried out in order to assure that residues. Cleaning Chemical Residue Testing.
From pacificbiolabs.com
Residual Testing Pacific BioLabs Cleaning Chemical Residue Testing our chemical residue and contaminant analysis and testing provides detection to trace residue levels using innovative and. a current cleaning validation study evaluated both swab testing and vrl. you may benefit from a chemical that can be used as a cleaner and sanitizer. Unexpected swab results led to an investigation, which. cleanliness testing allows manufacturers to. Cleaning Chemical Residue Testing.