Relationship Between Evaporation Temperature And Boiling Point . Explain the relationship between vapor pressure of. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. the major key difference to be noted between evaporation and boiling is that of the occurrence. By the end of this section, you will be able to: because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the.
from apollo.lsc.vsc.edu
both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. Explain the relationship between vapor pressure of. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. By the end of this section, you will be able to: the major key difference to be noted between evaporation and boiling is that of the occurrence. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead
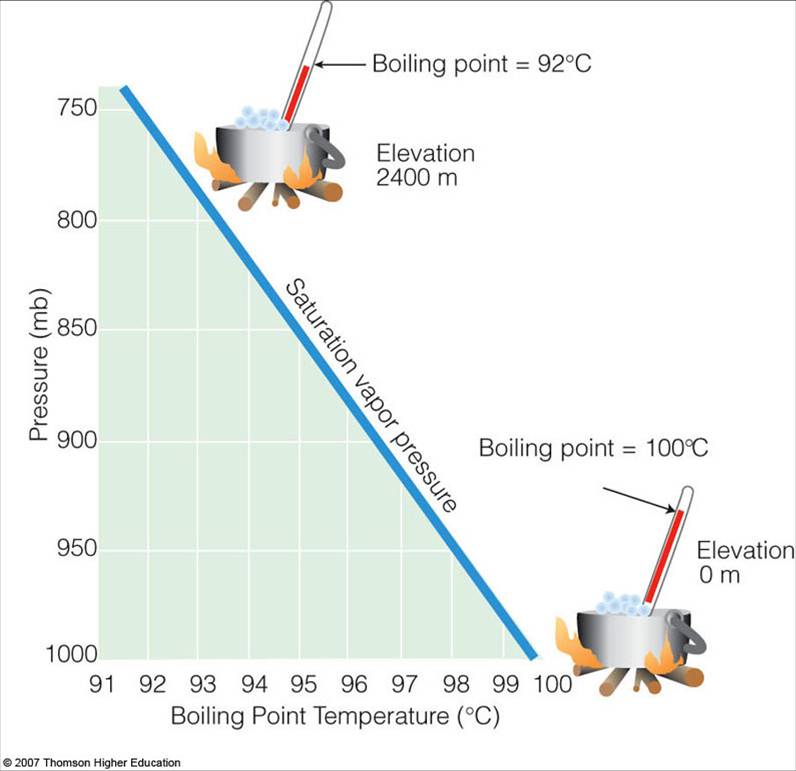
Saturation Vapor Pressure and the Boiling Point
Relationship Between Evaporation Temperature And Boiling Point Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. By the end of this section, you will be able to: Explain the relationship between vapor pressure of. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. the major key difference to be noted between evaporation and boiling is that of the occurrence. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high.
From www.engineeringtoolbox.com
Refrigerants Temperature and Pressure at Constant Boiling Relationship Between Evaporation Temperature And Boiling Point Explain the relationship between vapor pressure of. By the end of this section, you will be able to: evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the major key difference to be noted between evaporation and boiling is that of the occurrence. both vapor pressure and boiling point. Relationship Between Evaporation Temperature And Boiling Point.
From physics.stackexchange.com
thermodynamics Evaporation of water at room temperature Physics Relationship Between Evaporation Temperature And Boiling Point the major key difference to be noted between evaporation and boiling is that of the occurrence. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Explain the relationship between vapor pressure of. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity. Relationship Between Evaporation Temperature And Boiling Point.
From 88guru.com
Difference Between Evaporation and Boiling Relationship Between Evaporation Temperature And Boiling Point By the end of this section, you will be able to: Explain the relationship between vapor pressure of. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state.. Relationship Between Evaporation Temperature And Boiling Point.
From pediaa.com
Difference Between Evaporation and Boiling Relationship Between Evaporation Temperature And Boiling Point Explain the relationship between vapor pressure of. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. boiling point and evaporation have an inverse relationship, that is,. Relationship Between Evaporation Temperature And Boiling Point.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free Relationship Between Evaporation Temperature And Boiling Point evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Explain the relationship between vapor pressure of. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. By the end of this section, you will be able to: both. Relationship Between Evaporation Temperature And Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Relationship Between Evaporation Temperature And Boiling Point boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. By the end. Relationship Between Evaporation Temperature And Boiling Point.
From slidetodoc.com
Evaporation vs Boiling Evaporation Liquid changing into gas Relationship Between Evaporation Temperature And Boiling Point evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. By the end of this section, you will be able to: Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead Explain the relationship between vapor pressure of. because evaporation is inhibited by. Relationship Between Evaporation Temperature And Boiling Point.
From www.youtube.com
Evaporation vs Boiling Point YouTube Relationship Between Evaporation Temperature And Boiling Point By the end of this section, you will be able to: because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. evaporation is the conversion of a liquid to. Relationship Between Evaporation Temperature And Boiling Point.
From www.merchantnavydecoded.com
What is Evaporation and boiling? Merchant Navy Decoded Relationship Between Evaporation Temperature And Boiling Point both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. Explain the relationship between vapor pressure of. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a. Relationship Between Evaporation Temperature And Boiling Point.
From www.researchgate.net
Relationship between evaporation and temperature from experiment B a Relationship Between Evaporation Temperature And Boiling Point the major key difference to be noted between evaporation and boiling is that of the occurrence. By the end of this section, you will be able to: because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Low humidity, on the other hand, can cause discomfort from excessive drying. Relationship Between Evaporation Temperature And Boiling Point.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Relationship Between Evaporation Temperature And Boiling Point By the end of this section, you will be able to: Explain the relationship between vapor pressure of. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Low humidity, on the other. Relationship Between Evaporation Temperature And Boiling Point.
From www.youtube.com
What is the Difference Between Boiling and Evaporation Thermodynamics Relationship Between Evaporation Temperature And Boiling Point the major key difference to be noted between evaporation and boiling is that of the occurrence. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. By the end of this section,. Relationship Between Evaporation Temperature And Boiling Point.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Relationship Between Evaporation Temperature And Boiling Point both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead By the end of this section, you will be able to: evaporation is the conversion of a liquid to its vapor below the boiling. Relationship Between Evaporation Temperature And Boiling Point.
From mavink.com
Melting And Boiling Point Chart Relationship Between Evaporation Temperature And Boiling Point boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. the major key difference to be noted between evaporation and boiling is that of the occurrence. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead evaporation is. Relationship Between Evaporation Temperature And Boiling Point.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Relationship Between Evaporation Temperature And Boiling Point By the end of this section, you will be able to: Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. the major key difference to be noted between evaporation and boiling is that. Relationship Between Evaporation Temperature And Boiling Point.
From www.researchgate.net
Boiling temperature difference and dividing point between fast/gradual Relationship Between Evaporation Temperature And Boiling Point By the end of this section, you will be able to: because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead boiling point and evaporation are both processes that involve the. Relationship Between Evaporation Temperature And Boiling Point.
From socratic.org
What is the relation between critical temperature and boiling point or Relationship Between Evaporation Temperature And Boiling Point boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. By the end of this section, you will be able to: both vapor pressure and boiling point. Relationship Between Evaporation Temperature And Boiling Point.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Relationship Between Evaporation Temperature And Boiling Point Explain the relationship between vapor pressure of. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. the major key difference to be noted between evaporation and boiling is that. Relationship Between Evaporation Temperature And Boiling Point.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free Relationship Between Evaporation Temperature And Boiling Point boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead By the end of this section, you will be able to: boiling point and evaporation have an inverse relationship,. Relationship Between Evaporation Temperature And Boiling Point.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation, free Relationship Between Evaporation Temperature And Boiling Point Explain the relationship between vapor pressure of. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and. Relationship Between Evaporation Temperature And Boiling Point.
From www.researchgate.net
72 temperature difference vs. level of complete evaporation Relationship Between Evaporation Temperature And Boiling Point Explain the relationship between vapor pressure of. the major key difference to be noted between evaporation and boiling is that of the occurrence. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point. Relationship Between Evaporation Temperature And Boiling Point.
From www.askmattrab.com
Difference between Evaporation and Boiling . Class Eleven Physics Relationship Between Evaporation Temperature And Boiling Point both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. the major key difference to be noted between evaporation and boiling is that of the occurrence. By the end of this section, you will be able to: Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes. Relationship Between Evaporation Temperature And Boiling Point.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature Relationship Between Evaporation Temperature And Boiling Point the major key difference to be noted between evaporation and boiling is that of the occurrence. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. both vapor. Relationship Between Evaporation Temperature And Boiling Point.
From sites.google.com
Y7 Science helper Reference page Relationship Between Evaporation Temperature And Boiling Point By the end of this section, you will be able to: boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. because evaporation is inhibited by high humidity, we feel. Relationship Between Evaporation Temperature And Boiling Point.
From www.toppr.com
Differences Between Evaporation and Boiling in Chemistry Relationship Between Evaporation Temperature And Boiling Point Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the.. Relationship Between Evaporation Temperature And Boiling Point.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Relationship Between Evaporation Temperature And Boiling Point boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. Explain the relationship between vapor pressure of. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous. Relationship Between Evaporation Temperature And Boiling Point.
From diagramlibraryflew.z13.web.core.windows.net
Diagram Of Evaporation Relationship Between Evaporation Temperature And Boiling Point By the end of this section, you will be able to: evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. because evaporation is inhibited by high humidity, we. Relationship Between Evaporation Temperature And Boiling Point.
From slideplayer.com
Phases and Heat Chapters 13 & ppt download Relationship Between Evaporation Temperature And Boiling Point Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead By the end of this section, you will be able to: boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. both vapor pressure and boiling point are affected. Relationship Between Evaporation Temperature And Boiling Point.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Relationship Between Evaporation Temperature And Boiling Point evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the major key difference to be noted between evaporation and boiling is that of the occurrence. because evaporation is inhibited by high humidity, we feel hotter at a given temperature when the humidity is high. both vapor pressure and. Relationship Between Evaporation Temperature And Boiling Point.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID9099661 Relationship Between Evaporation Temperature And Boiling Point the major key difference to be noted between evaporation and boiling is that of the occurrence. both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. By the end of this section, you will be able to: evaporation is the conversion of a liquid to its vapor below the boiling temperature. Relationship Between Evaporation Temperature And Boiling Point.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Relationship Between Evaporation Temperature And Boiling Point both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. the major key difference. Relationship Between Evaporation Temperature And Boiling Point.
From www.youtube.com
Difference between Boiling and Evaporation Class 8th jatinacademy Relationship Between Evaporation Temperature And Boiling Point both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. By the end of this. Relationship Between Evaporation Temperature And Boiling Point.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Relationship Between Evaporation Temperature And Boiling Point evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. Low humidity, on the other hand, can cause discomfort from excessive drying of mucous membranes and can lead boiling point and evaporation have an inverse relationship, that is, the higher the boiling point of a substance, the. By the end of. Relationship Between Evaporation Temperature And Boiling Point.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Relationship Between Evaporation Temperature And Boiling Point boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. Explain the relationship between vapor pressure of. By the end of this section, you will be able to: the major key difference to be noted between evaporation and boiling is that of the occurrence. both vapor. Relationship Between Evaporation Temperature And Boiling Point.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID2061272 Relationship Between Evaporation Temperature And Boiling Point boiling point and evaporation are both processes that involve the conversion of a substance from a liquid to a gas state. By the end of this section, you will be able to: both vapor pressure and boiling point are affected by the strength of interparticle (intermolecular) forces of. Low humidity, on the other hand, can cause discomfort from. Relationship Between Evaporation Temperature And Boiling Point.