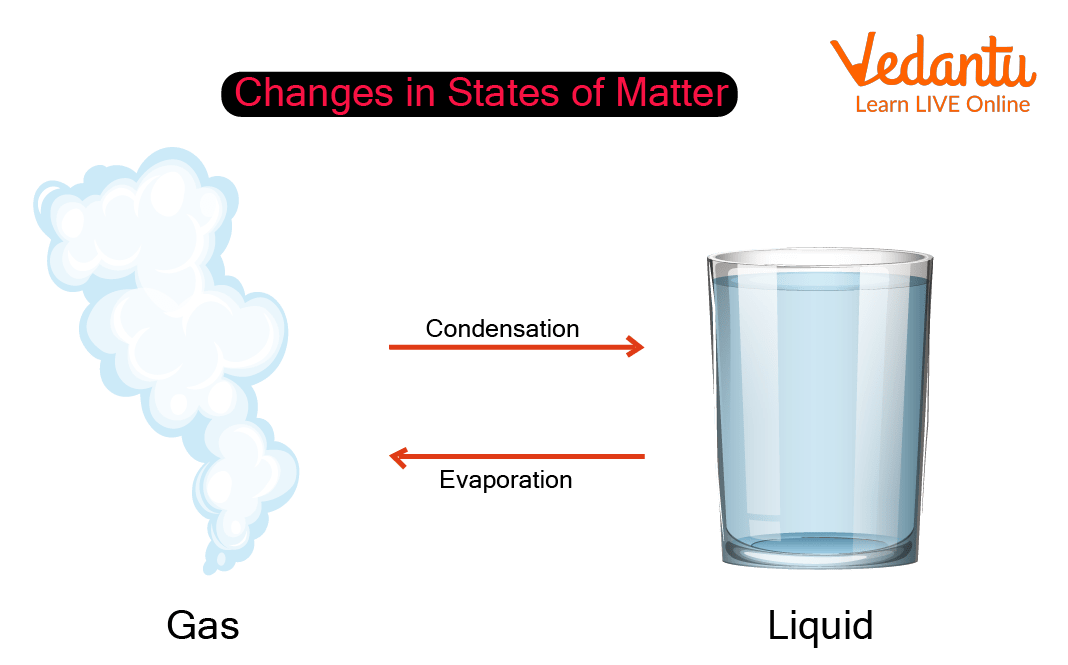
Example For Condensation Gas To Liquid . Explain that as water molecules in the air cool and slow down, their attractions overcome. Latent heat of vaporization (boiling or condensation): The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. In condensation, a gas (like water vapor) changes state to become a liquid (water). The liquefaction of gases is a complicated process that uses various compressions and. While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form.
from www.vedantu.com
Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. In condensation, a gas (like water vapor) changes state to become a liquid (water). Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and. Latent heat of vaporization (boiling or condensation): Explain that as water molecules in the air cool and slow down, their attractions overcome.
Evaporation and Condensation Definition, Applications and Difference
Example For Condensation Gas To Liquid The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. In condensation, a gas (like water vapor) changes state to become a liquid (water). Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Explain that as water molecules in the air cool and slow down, their attractions overcome. The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and. Latent heat of vaporization (boiling or condensation): While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form.
From slideplayer.com
Matter. ppt download Example For Condensation Gas To Liquid Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Explain that as water molecules in the air cool and slow down, their attractions overcome. The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. In condensation, a gas (like water vapor) changes. Example For Condensation Gas To Liquid.
From mavink.com
Condensation Diagram Example For Condensation Gas To Liquid Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Explain that as water molecules in the air cool and slow down, their attractions overcome. Latent heat of vaporization (boiling or condensation): While condensation occurs when a gas or vapor changes into liquid form,. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Phase Change PowerPoint Presentation, free download ID6850110 Example For Condensation Gas To Liquid In condensation, a gas (like water vapor) changes state to become a liquid (water). Explain that as water molecules in the air cool and slow down, their attractions overcome. Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The amount of heat energy required to change the state of a substance from a liquid to. Example For Condensation Gas To Liquid.
From www.snexplores.org
Explainer What are the different states of matter? Example For Condensation Gas To Liquid In condensation, a gas (like water vapor) changes state to become a liquid (water). Explain that as water molecules in the air cool and slow down, their attractions overcome. While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. Condensation commonly occurs when a vapor is cooled and/or. Example For Condensation Gas To Liquid.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Example For Condensation Gas To Liquid Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Explain that as water molecules in the air cool and slow down, their attractions overcome. Latent heat of vaporization (boiling or condensation): The liquefaction of gases is a complicated process that uses various compressions and. While condensation occurs when a gas or vapor changes into liquid. Example For Condensation Gas To Liquid.
From stock.adobe.com
Vector scientific illustration of changing states of matter from liquid Example For Condensation Gas To Liquid Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and. Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Explain that as water molecules in the. Example For Condensation Gas To Liquid.
From slideplayer.com
EVAPORATION, CONDENSATION, PRECIPITATION AND TRANSPIRATION ppt download Example For Condensation Gas To Liquid Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Latent heat of vaporization (boiling or condensation): Explain that as water molecules in the air cool and slow down, their attractions overcome. Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID9099661 Example For Condensation Gas To Liquid Latent heat of vaporization (boiling or condensation): Explain that as water molecules in the air cool and slow down, their attractions overcome. Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. In condensation, a gas (like water vapor) changes state to become a. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT condensation PowerPoint Presentation, free download ID2171352 Example For Condensation Gas To Liquid The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. The liquefaction of gases is a complicated process that uses various compressions and. Explain that. Example For Condensation Gas To Liquid.
From dokumen.tips
(PDF) Condensation (Gas to Liquid)hydrogen bonds. Predicting Types of Example For Condensation Gas To Liquid Latent heat of vaporization (boiling or condensation): Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Explain that as water molecules in the air cool and slow down, their attractions overcome. While condensation occurs when a gas or vapor changes into liquid form,. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Ch. 3.1 Solids, Liquids, Gases PowerPoint Presentation, free Example For Condensation Gas To Liquid Explain that as water molecules in the air cool and slow down, their attractions overcome. Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. In condensation, a gas (like water vapor) changes state to become a liquid (water). While condensation occurs when a. Example For Condensation Gas To Liquid.
From www.youtube.com
How Does Water Go From A Gas To A Liquid? Condensation YouTube Example For Condensation Gas To Liquid Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. In condensation, a gas (like water vapor) changes state to become a liquid (water). While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to. Example For Condensation Gas To Liquid.
From kids.britannica.com
evaporation and condensation Kids Britannica Kids Homework Help Example For Condensation Gas To Liquid Explain that as water molecules in the air cool and slow down, their attractions overcome. The liquefaction of gases is a complicated process that uses various compressions and. In condensation, a gas (like water vapor) changes state to become a liquid (water). Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Latent heat of vaporization. Example For Condensation Gas To Liquid.
From www.exampleslab.com
16 examples of gastoliquid condensation and common uses Examples Lab Example For Condensation Gas To Liquid Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. In condensation, a gas (like water vapor) changes state to become a liquid (water). While condensation occurs when a gas. Example For Condensation Gas To Liquid.
From slideshare.net
The Water Cycle Example For Condensation Gas To Liquid Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Explain that as water molecules in the air cool and slow down, their attractions overcome. Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Latent heat of vaporization (boiling. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 Example For Condensation Gas To Liquid Latent heat of vaporization (boiling or condensation): The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. The liquefaction of gases. Example For Condensation Gas To Liquid.
From slideplayer.com
Chapter 3 States of Matter. ppt download Example For Condensation Gas To Liquid The liquefaction of gases is a complicated process that uses various compressions and. While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. Latent heat. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT KMT and Gas Laws PowerPoint Presentation, free download ID2233865 Example For Condensation Gas To Liquid Explain that as water molecules in the air cool and slow down, their attractions overcome. While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID6053479 Example For Condensation Gas To Liquid Latent heat of vaporization (boiling or condensation): Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. While condensation occurs when a gas or vapor changes into liquid form, evaporation. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Physical Science PowerPoint Presentation, free download ID4307121 Example For Condensation Gas To Liquid Explain that as water molecules in the air cool and slow down, their attractions overcome. In condensation, a gas (like water vapor) changes state to become a liquid (water). The liquefaction of gases is a complicated process that uses various compressions and. Latent heat of vaporization (boiling or condensation): Condensation commonly occurs when a vapor is cooled and/or compressed to. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation, free Example For Condensation Gas To Liquid Explain that as water molecules in the air cool and slow down, their attractions overcome. Latent heat of vaporization (boiling or condensation): Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Liquefaction of gases is physical conversion of a gas into a liquid. Example For Condensation Gas To Liquid.
From www.youtube.com
Factors Affecting the Rate of Condensation Turning a Gas into a Example For Condensation Gas To Liquid Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Latent heat of vaporization (boiling or condensation): In condensation, a gas (like water vapor) changes state to become a liquid (water). The liquefaction of gases is a complicated process that uses various compressions and.. Example For Condensation Gas To Liquid.
From slideplayer.com
CHAPTER 3 LESSON 2 SYSTEM INTERACTIONS. ppt download Example For Condensation Gas To Liquid In condensation, a gas (like water vapor) changes state to become a liquid (water). Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. While condensation occurs when a gas. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Topic Chemistry PowerPoint Presentation, free download ID5826930 Example For Condensation Gas To Liquid Latent heat of vaporization (boiling or condensation): While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. In condensation, a gas (like water vapor) changes. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID2814115 Example For Condensation Gas To Liquid Explain that as water molecules in the air cool and slow down, their attractions overcome. The liquefaction of gases is a complicated process that uses various compressions and. In condensation, a gas (like water vapor) changes state to become a liquid (water). The amount of heat energy required to change the state of a substance from a liquid to a. Example For Condensation Gas To Liquid.
From sciencenotes.org
Examples of Gases What Is a Gas? Example For Condensation Gas To Liquid In condensation, a gas (like water vapor) changes state to become a liquid (water). The liquefaction of gases is a complicated process that uses various compressions and. The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. Explain that as water molecules in the air cool and. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT MATTER PowerPoint Presentation, free download ID1134662 Example For Condensation Gas To Liquid While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. Latent heat of vaporization (boiling or condensation): The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. The liquefaction of gases is a complicated process. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3746915 Example For Condensation Gas To Liquid Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). In condensation, a gas (like water vapor) changes state to become a liquid (water). The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. Condensation commonly occurs when a vapor is cooled and/or. Example For Condensation Gas To Liquid.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Example For Condensation Gas To Liquid Explain that as water molecules in the air cool and slow down, their attractions overcome. Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. The amount of heat energy required to change the state of a substance from a liquid to a gas. Example For Condensation Gas To Liquid.
From www.vedantu.com
Evaporation and Condensation Definition, Applications and Difference Example For Condensation Gas To Liquid Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. In condensation, a gas (like water vapor) changes state to become. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free Example For Condensation Gas To Liquid Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. Explain that as water molecules in the air cool and slow down, their attractions overcome. The amount of heat energy required to change the state of a substance from a liquid to a gas. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT Phase/State Changes PowerPoint Presentation, free download ID Example For Condensation Gas To Liquid Latent heat of vaporization (boiling or condensation): While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). Explain that as water molecules in the air cool and slow down, their attractions overcome. The liquefaction. Example For Condensation Gas To Liquid.
From www.slideserve.com
PPT How do particles behave in the four states of matter? PowerPoint Example For Condensation Gas To Liquid The liquefaction of gases is a complicated process that uses various compressions and. Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to gaseous form. Condensation commonly occurs when a vapor is cooled and/or compressed to. Example For Condensation Gas To Liquid.
From www.britannica.com
Phase Definition & Facts Britannica Example For Condensation Gas To Liquid In condensation, a gas (like water vapor) changes state to become a liquid (water). The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at. The liquefaction of gases is a complicated process that uses various compressions and. Explain that as water molecules in the air cool and. Example For Condensation Gas To Liquid.
From www.youtube.com
CONDENSATION CHANGING OF MATTER (GAS LIQUID) SCIENCE 3 QUARTER 1 Example For Condensation Gas To Liquid In condensation, a gas (like water vapor) changes state to become a liquid (water). Condensation commonly occurs when a vapor is cooled and/or compressed to its saturation limit when the molecular density in the gas phase reaches its maximal threshold. While condensation occurs when a gas or vapor changes into liquid form, evaporation happens when a liquid substance changes to. Example For Condensation Gas To Liquid.