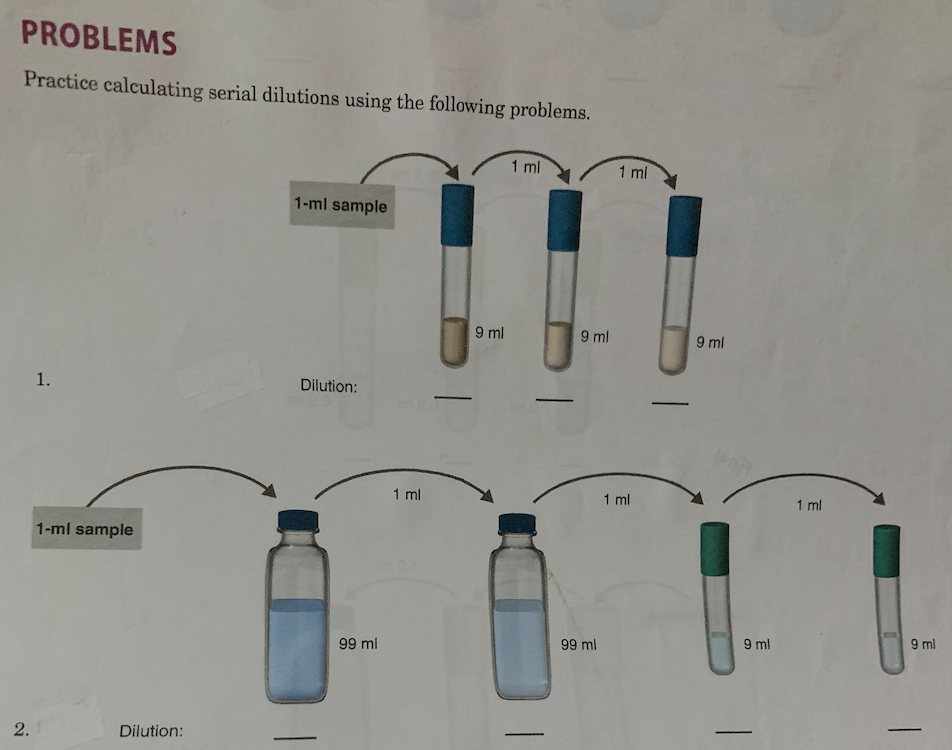
Serial Dilutions Practice Problems . The following problem sets test your ability to calculate dilution factors and concentration*s. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. The following problem sets test your ability to calculate dilution factors and concentration*s. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. You do three 1:100 serial dilutions on an actively. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. Use three significant figures for your final answer (e.g. Individual dilution factors at each tube tube 1:
from www.chegg.com
If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Use three significant figures for your final answer (e.g. The following problem sets test your ability to calculate dilution factors and concentration*s. Individual dilution factors at each tube tube 1: If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. You do three 1:100 serial dilutions on an actively.
Solved PROBLEMS Practice calculating serial dilutions using
Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. The following problem sets test your ability to calculate dilution factors and concentration*s. You do three 1:100 serial dilutions on an actively. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. Use three significant figures for your final answer (e.g. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Individual dilution factors at each tube tube 1: The following problem sets test your ability to calculate dilution factors and concentration*s.
From answerlistsybil.z13.web.core.windows.net
Dilution Practice Problems Worksheet Answers Serial Dilutions Practice Problems Individual dilution factors at each tube tube 1: If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with. Serial Dilutions Practice Problems.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Individual dilution factors at each tube tube 1: The following problem sets test your ability to calculate dilution factors and concentration*s. If 1 ml sample. Serial Dilutions Practice Problems.
From www.chegg.com
Solved Design and draw a serial dilution to go from 5 times Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. Individual dilution factors at each tube. Serial Dilutions Practice Problems.
From www.slideshare.net
Serial dilution Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. You do three 1:100 serial dilutions on an actively. If you dilute 175 ml. Serial Dilutions Practice Problems.
From prabhakarpk.blogspot.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. You do three 1:100 serial dilutions on an actively. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Use three significant figures for your final answer (e.g. Most specimens have high enough numbers of. Serial Dilutions Practice Problems.
From materialzonetomlin.z21.web.core.windows.net
Dilution Problems Worksheets Serial Dilutions Practice Problems Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. The following problem sets test your ability to calculate dilution factors and concentration*s. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l,. Serial Dilutions Practice Problems.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Serial Dilutions Practice Problems Use three significant figures for your final answer (e.g. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate. Serial Dilutions Practice Problems.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Serial Dilutions Practice Problems You do three 1:100 serial dilutions on an actively. The following problem sets test your ability to calculate dilution factors and concentration*s. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with. Serial Dilutions Practice Problems.
From www.chegg.com
SERIAL DILUTIONS PRACTICE PROBLEMO If the final Serial Dilutions Practice Problems Individual dilution factors at each tube tube 1: You do three 1:100 serial dilutions on an actively. Use three significant figures for your final answer (e.g. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the. Serial Dilutions Practice Problems.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Serial Dilutions Practice Problems If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. The following problem sets test your ability to calculate dilution factors and concentration*s. Use three significant figures for your final answer (e.g. Most specimens have high enough. Serial Dilutions Practice Problems.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Serial Dilutions Practice Problems Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Individual dilution factors at each tube tube 1: The following problem sets test your ability to calculate dilution factors. Serial Dilutions Practice Problems.
From www.chegg.com
Solved PROBLEMS Practice calculating serial dilutions using Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. Use three significant figures for your final answer (e.g. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. If you dilute 175 ml. Serial Dilutions Practice Problems.
From www.chegg.com
Solved Practice Dilution Problems Section I 1. How much Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. Use three significant figures for your final answer (e.g. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. Individual dilution factors at each tube tube 1: The following problem sets test your ability to calculate dilution factors. Serial Dilutions Practice Problems.
From studylib.net
Lab 28 notes Dilution Series Problems and Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Individual dilution factors at each tube tube 1: If 1 ml sample. Serial Dilutions Practice Problems.
From www.coursehero.com
[Solved] Design a serial dilution to achieve a final dilution of 10^8 Serial Dilutions Practice Problems Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. The following problem sets test your ability to calculate dilution factors and concentration*s. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution. Serial Dilutions Practice Problems.
From www.youtube.com
Dilution Calculation Practice YouTube Serial Dilutions Practice Problems If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0. Serial Dilutions Practice Problems.
From www.chegg.com
Solved PRACTICE SERIAL DILUTION PROBLEMS Assume the Serial Dilutions Practice Problems Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. Individual dilution factors at each tube tube 1: The following problem sets test your ability to calculate dilution factors and concentration*s. The following problem sets test your ability to calculate dilution factors and concentration*s. If 1 ml sample from tube #1. Serial Dilutions Practice Problems.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Serial Dilutions Practice Problems Use three significant figures for your final answer (e.g. You do three 1:100 serial dilutions on an actively. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. The following problem sets test your ability to calculate. Serial Dilutions Practice Problems.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Serial Dilutions Practice Problems If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0. Serial Dilutions Practice Problems.
From www.chegg.com
Solved Dilution practice problems 1. 'Calculate CFU/ml of Serial Dilutions Practice Problems You do three 1:100 serial dilutions on an actively. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. Use three significant figures for your final answer (e.g. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution. Serial Dilutions Practice Problems.
From renewmodern.weebly.com
Serial Dilution Explained renewmodern Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. The following problem sets test your ability to calculate dilution factors and concentration*s. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. You do three 1:100 serial dilutions on an actively. If you dilute 175 ml of. Serial Dilutions Practice Problems.
From treeprecision.weebly.com
3 Fold Serial Dilution treeprecision Serial Dilutions Practice Problems If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. If 1 ml sample from tube #1 with. Serial Dilutions Practice Problems.
From printablejaycee99.s3-website-us-east-1.amazonaws.com
Serial Dilutions Worksheet Serial Dilutions Practice Problems You do three 1:100 serial dilutions on an actively. Individual dilution factors at each tube tube 1: The following problem sets test your ability to calculate dilution factors and concentration*s. The following problem sets test your ability to calculate dilution factors and concentration*s. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube. Serial Dilutions Practice Problems.
From www.youtube.com
Example Problems SPC and Serial Dilutions YouTube Serial Dilutions Practice Problems Use three significant figures for your final answer (e.g. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. You do three 1:100 serial dilutions on an actively. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent,. Serial Dilutions Practice Problems.
From www.chegg.com
Solved Serial Dilution Homework Problems 1 In the image Serial Dilutions Practice Problems Individual dilution factors at each tube tube 1: If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively.. Serial Dilutions Practice Problems.
From gambr.co
️Serial Dilution Practice Problems Worksheet Free Download Gambr.co Serial Dilutions Practice Problems Use three significant figures for your final answer (e.g. The following problem sets test your ability to calculate dilution factors and concentration*s. You do three 1:100 serial dilutions on an actively. The following problem sets test your ability to calculate dilution factors and concentration*s. Individual dilution factors at each tube tube 1: Most specimens have high enough numbers of microorganisms. Serial Dilutions Practice Problems.
From www.youtube.com
Dilution Practice Problems 4 & 5 YouTube Serial Dilutions Practice Problems If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. The following problem sets test. Serial Dilutions Practice Problems.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Serial Dilutions Practice Problems Individual dilution factors at each tube tube 1: Use three significant figures for your final answer (e.g. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. You do three 1:100 serial dilutions on an actively. If 1 ml sample from tube #1 with a tube dilution of. Serial Dilutions Practice Problems.
From view.icsl.edu.gr
Serial Dilution Worksheet Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. If 1 ml sample from tube. Serial Dilutions Practice Problems.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Serial Dilutions Practice Problems If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. If 1 ml sample form tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. The following problem sets test. Serial Dilutions Practice Problems.
From worksheets.ekocraft-appleleaf.com
Serial Dilution Worksheet Worksheets For Kindergarten Serial Dilutions Practice Problems If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0. Serial Dilutions Practice Problems.
From www.chegg.com
Solved For the following serial dilution fill in the blanks, Serial Dilutions Practice Problems Use three significant figures for your final answer (e.g. The following problem sets test your ability to calculate dilution factors and concentration*s. If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. Individual dilution factors at each. Serial Dilutions Practice Problems.
From www.chegg.com
Solved SERIAL DILUTIONS PRACTICE PROBLEMS If t. other three Serial Dilutions Practice Problems Use three significant figures for your final answer (e.g. Individual dilution factors at each tube tube 1: If 1 ml sample from tube #1 with a tube dilution of 1/25 is placed in tube #2 with 24 ml of diluent, what is the tube dilution and what is the final. If 1 ml sample form tube #1 with a tube. Serial Dilutions Practice Problems.
From davida.davivienda.com
Dilution Practice Problems Worksheet Answers Printable Word Searches Serial Dilutions Practice Problems The following problem sets test your ability to calculate dilution factors and concentration*s. Individual dilution factors at each tube tube 1: Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. You do three 1:100 serial dilutions on an actively. Use three significant figures for your final answer (e.g. The following. Serial Dilutions Practice Problems.
From www.chegg.com
Solved Please try to solve this serial dilution problem and Serial Dilutions Practice Problems Most specimens have high enough numbers of microorganisms that the specimen has to be serially diluted to quantitate effectively. The following problem sets test your ability to calculate dilution factors and concentration*s. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. If 1 ml sample form tube. Serial Dilutions Practice Problems.