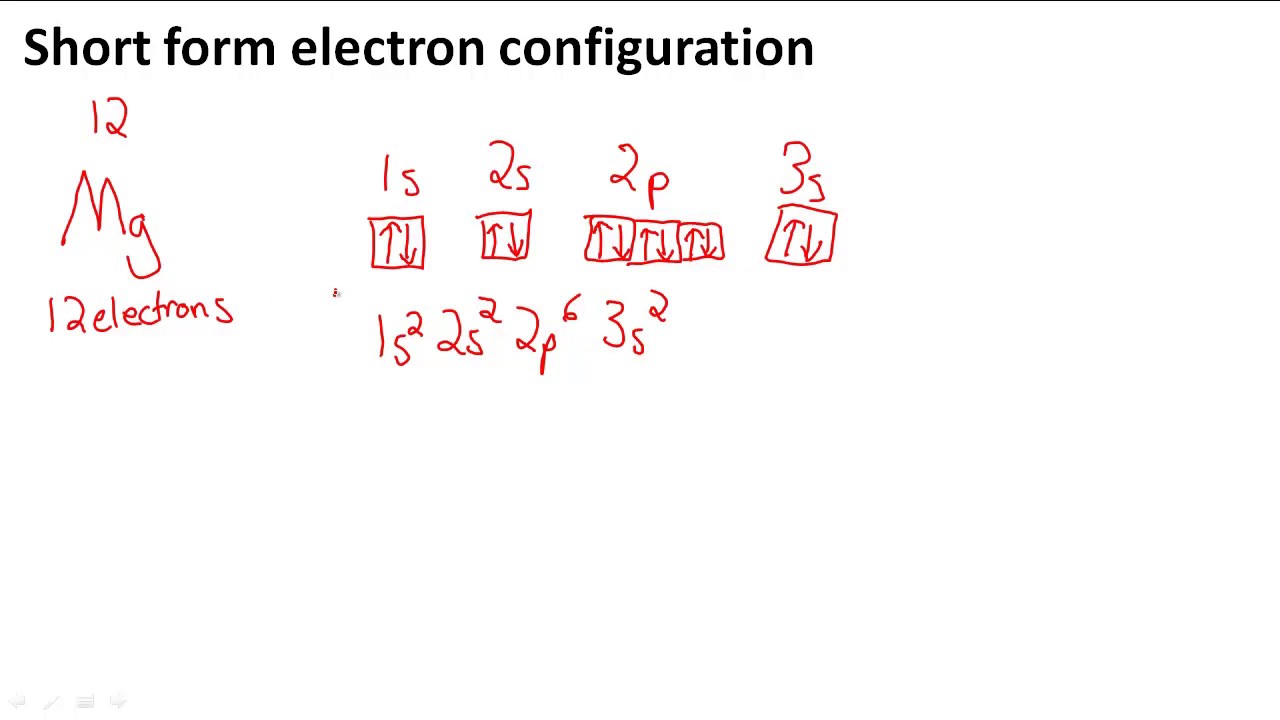
Electron Configuration Of Neutral Magnesium . In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Magnesium occurs naturally only in combination with other. Electron configuration of magnesium is [ne] 3s2. Electron configuration chart of all elements is mentioned in the table below. Ne 3s2 is the electron configuration for mg. Many other electrons configurations of other elements have been provided here for the user. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas. You can check it here: Possible oxidation states are +2. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne).
from utedzz.blogspot.com
The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Electron configuration chart of all elements is mentioned in the table below. Many other electrons configurations of other elements have been provided here for the user. Ne 3s2 is the electron configuration for mg. Magnesium occurs naturally only in combination with other. Possible oxidation states are +2. You can check it here: Electron configuration of magnesium is [ne] 3s2. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas.
Periodic Table Magnesium Electron Configuration Periodic Table Timeline
Electron Configuration Of Neutral Magnesium This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Possible oxidation states are +2. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Many other electrons configurations of other elements have been provided here for the user. Magnesium occurs naturally only in combination with other. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of magnesium is [ne] 3s2. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Ne 3s2 is the electron configuration for mg. You can check it here: The shorthand electron configuration (or noble gas. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element.
From www.youtube.com
Magnesium Ground State Electron Configuration YouTube Electron Configuration Of Neutral Magnesium You can check it here: Electron configuration chart of all elements is mentioned in the table below. Possible oxidation states are +2. Electron configuration of magnesium is [ne] 3s2. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The shorthand electron configuration (or noble. Electron Configuration Of Neutral Magnesium.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Neutral Magnesium The shorthand electron configuration (or noble gas. Magnesium occurs naturally only in combination with other. You can check it here: In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Ne 3s2 is the electron configuration for mg. Electron configuration chart of all elements is. Electron Configuration Of Neutral Magnesium.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Electron Configuration Of Neutral Magnesium Magnesium occurs naturally only in combination with other. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration of magnesium is [ne] 3s2. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble. Electron Configuration Of Neutral Magnesium.
From galvinconanstuart.blogspot.com
Construct An Orbital Diagram To Show The Electron Configuration For A Electron Configuration Of Neutral Magnesium Many other electrons configurations of other elements have been provided here for the user. The shorthand electron configuration (or noble gas. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Ne 3s2 is the electron configuration for mg. Electron configuration chart of all elements. Electron Configuration Of Neutral Magnesium.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Neutral Magnesium You can check it here: Ne 3s2 is the electron configuration for mg. Possible oxidation states are +2. Many other electrons configurations of other elements have been provided here for the user. Electron configuration chart of all elements is mentioned in the table below. Electron configuration of magnesium is [ne] 3s2. In order to write the mg electron configuration we. Electron Configuration Of Neutral Magnesium.
From wiringdatabaseinfo.blogspot.com
Construct An Orbital Diagram To Show The Electron Configuration For A Electron Configuration Of Neutral Magnesium You can check it here: In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration chart of all elements is mentioned in the table below. Ne 3s2 is the electron configuration for mg. Many other electrons configurations of other elements have been provided. Electron Configuration Of Neutral Magnesium.
From www.numerade.com
SOLVED Draw the electron configuration for a neutral atom of magnesium Electron Configuration Of Neutral Magnesium Ne 3s2 is the electron configuration for mg. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Magnesium occurs naturally only in combination with other. Possible oxidation. Electron Configuration Of Neutral Magnesium.
From galvinconanstuart.blogspot.com
Construct An Orbital Diagram To Show The Electron Configuration For A Electron Configuration Of Neutral Magnesium You can check it here: The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Many other electrons configurations of other elements have been provided here for the user. Electron configuration of magnesium is [ne] 3s2. Magnesium occurs naturally only in combination with other. Possible oxidation states. Electron Configuration Of Neutral Magnesium.
From www.webelements.com
Elements Periodic Table » Magnesium » properties of free atoms Electron Configuration Of Neutral Magnesium In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Possible oxidation states are +2. Magnesium occurs naturally only in combination with other. Electron configuration chart of all elements is mentioned in the table below. You can check it here: Many other electrons configurations of. Electron Configuration Of Neutral Magnesium.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Electron Configuration Of Neutral Magnesium This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Electron configuration of magnesium is [ne] 3s2. Ne 3s2 is the electron configuration for mg. Possible oxidation states. Electron Configuration Of Neutral Magnesium.
From wiringdatabaseinfo.blogspot.com
Construct An Orbital Diagram To Show The Electron Configuration For A Electron Configuration Of Neutral Magnesium In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). You can check it here: Many other electrons configurations of other elements have been provided here for the user. Possible oxidation states are +2. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that. Electron Configuration Of Neutral Magnesium.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Electron Configuration Of Neutral Magnesium The shorthand electron configuration (or noble gas. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration of magnesium is [ne] 3s2. You can check it here: In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there. Electron Configuration Of Neutral Magnesium.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Electron Configuration Of Neutral Magnesium Electron configuration chart of all elements is mentioned in the table below. Possible oxidation states are +2. Ne 3s2 is the electron configuration for mg. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Electron configuration of magnesium is [ne] 3s2. You can check it here:. Electron Configuration Of Neutral Magnesium.
From galvinconanstuart.blogspot.com
Magnesium Orbital Diagram General Wiring Diagram Electron Configuration Of Neutral Magnesium Electron configuration of magnesium is [ne] 3s2. Ne 3s2 is the electron configuration for mg. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). You can check it here: Many other electrons configurations of other elements have been provided here for the user. Electron configuration chart. Electron Configuration Of Neutral Magnesium.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Neutral Magnesium Electron configuration of magnesium is [ne] 3s2. Many other electrons configurations of other elements have been provided here for the user. You can check it here: Electron configuration chart of all elements is mentioned in the table below. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Magnesium occurs naturally. Electron Configuration Of Neutral Magnesium.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Electron Configuration Of Neutral Magnesium Possible oxidation states are +2. Ne 3s2 is the electron configuration for mg. Electron configuration of magnesium is [ne] 3s2. You can check it here: Electron configuration chart of all elements is mentioned in the table below. Many other electrons configurations of other elements have been provided here for the user. The electron configuration of mg2+ is 1s² 2s² 2p⁶,. Electron Configuration Of Neutral Magnesium.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Neutral Magnesium The shorthand electron configuration (or noble gas. Many other electrons configurations of other elements have been provided here for the user. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Electron configuration chart of all elements is mentioned in the table below. Ne 3s2 is the. Electron Configuration Of Neutral Magnesium.
From www.myxxgirl.com
Atom Diagram Of Magnesium Diagram To Show Ionic Bonding In Magnesium Electron Configuration Of Neutral Magnesium You can check it here: The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The shorthand electron configuration (or noble gas. Magnesium occurs naturally only in combination with other. Many other electrons configurations of other elements have been provided here for the user. Electron configuration chart. Electron Configuration Of Neutral Magnesium.
From www.newtondesk.com
magnesium electron configuration Newton Desk Electron Configuration Of Neutral Magnesium This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). You can check it here: Electron configuration of magnesium is [ne] 3s2. The shorthand electron configuration (or noble. Electron Configuration Of Neutral Magnesium.
From www.numerade.com
SOLVED Base your answers to questions 3032 on the information below Electron Configuration Of Neutral Magnesium Ne 3s2 is the electron configuration for mg. Many other electrons configurations of other elements have been provided here for the user. Magnesium occurs naturally only in combination with other. Possible oxidation states are +2. Electron configuration of magnesium is [ne] 3s2. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is. Electron Configuration Of Neutral Magnesium.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Electron Configuration Of Neutral Magnesium In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Magnesium occurs naturally only in combination with other. Many other electrons configurations of other elements have. Electron Configuration Of Neutral Magnesium.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents Electron Configuration Of Neutral Magnesium Electron configuration of magnesium is [ne] 3s2. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. You can check it here: The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Ne 3s2 is the electron configuration. Electron Configuration Of Neutral Magnesium.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Electron Configuration Of Neutral Magnesium Many other electrons configurations of other elements have been provided here for the user. Possible oxidation states are +2. You can check it here: In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration of magnesium is [ne] 3s2. The electron configuration of. Electron Configuration Of Neutral Magnesium.
From valenceelectrons.com
How to Find the Valence Electrons for Magnesium (Mg)? Electron Configuration Of Neutral Magnesium Possible oxidation states are +2. Magnesium occurs naturally only in combination with other. Ne 3s2 is the electron configuration for mg. The shorthand electron configuration (or noble gas. Electron configuration of magnesium is [ne] 3s2. You can check it here: In order to write the mg electron configuration we first need to know the number of electrons for the mg. Electron Configuration Of Neutral Magnesium.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Electron Configuration Of Neutral Magnesium This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. Many other electrons configurations of other elements have been provided here for the user. Ne 3s2 is the electron configuration for mg.. Electron Configuration Of Neutral Magnesium.
From www.chegg.com
Solved Construct an orbital diagram to show the electron Electron Configuration Of Neutral Magnesium Possible oxidation states are +2. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Many other electrons configurations of other elements have been provided here for the user. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron. Electron Configuration Of Neutral Magnesium.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Neutral Magnesium You can check it here: The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium occurs naturally only in combination with other. Many other electrons configurations of other elements have been provided here for the user. Electron configuration chart of all elements is mentioned in the. Electron Configuration Of Neutral Magnesium.
From www.chegg.com
Solved Construct an orbital diagram to show the electron Electron Configuration Of Neutral Magnesium In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). You can check it here: Many other electrons configurations of other elements have been provided here for the user. Magnesium occurs naturally only in combination with other. The shorthand electron configuration (or noble gas. This. Electron Configuration Of Neutral Magnesium.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Electron Configuration Of Neutral Magnesium Ne 3s2 is the electron configuration for mg. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium occurs naturally only in combination with other. You can check it here: Electron configuration chart of all elements is mentioned in the table below. Electron configuration of magnesium. Electron Configuration Of Neutral Magnesium.
From wirelistcollegium.z14.web.core.windows.net
Magnesium Orbital Diagram Electron Configuration Of Neutral Magnesium The shorthand electron configuration (or noble gas. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Many other electrons configurations of other elements have been provided here for the user. Ne 3s2 is the electron configuration for mg. Electron configuration of magnesium is [ne] 3s2. Electron. Electron Configuration Of Neutral Magnesium.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Electron Configuration Of Neutral Magnesium Electron configuration of magnesium is [ne] 3s2. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Possible oxidation states are +2. The shorthand electron configuration. Electron Configuration Of Neutral Magnesium.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Electron Configuration Of Neutral Magnesium In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Electron configuration of magnesium is [ne] 3s2. You can check it here: Ne 3s2 is the electron configuration for mg. Possible oxidation states are +2. Electron configuration chart of all elements is mentioned in the. Electron Configuration Of Neutral Magnesium.
From enginedatanichered.z21.web.core.windows.net
Mg Orbital Diagram Electron Configuration Of Neutral Magnesium Magnesium occurs naturally only in combination with other. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Electron configuration of magnesium is [ne] 3s2. Many other electrons configurations of other elements have been provided here for the user. Electron configuration chart of all elements is mentioned. Electron Configuration Of Neutral Magnesium.
From guidemanualeruptivity.z14.web.core.windows.net
Magnesium Mg Ground State Orbital Diagram Electron Configuration Of Neutral Magnesium The shorthand electron configuration (or noble gas. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Magnesium occurs naturally only in combination with other. You can check it here: Electron configuration of magnesium is. Electron Configuration Of Neutral Magnesium.
From www.chegg.com
Solved Draw the electron configuration for a neutral atom of Electron Configuration Of Neutral Magnesium In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Many other electrons configurations of other elements have been provided here for the user. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas. Electron Configuration Of Neutral Magnesium.