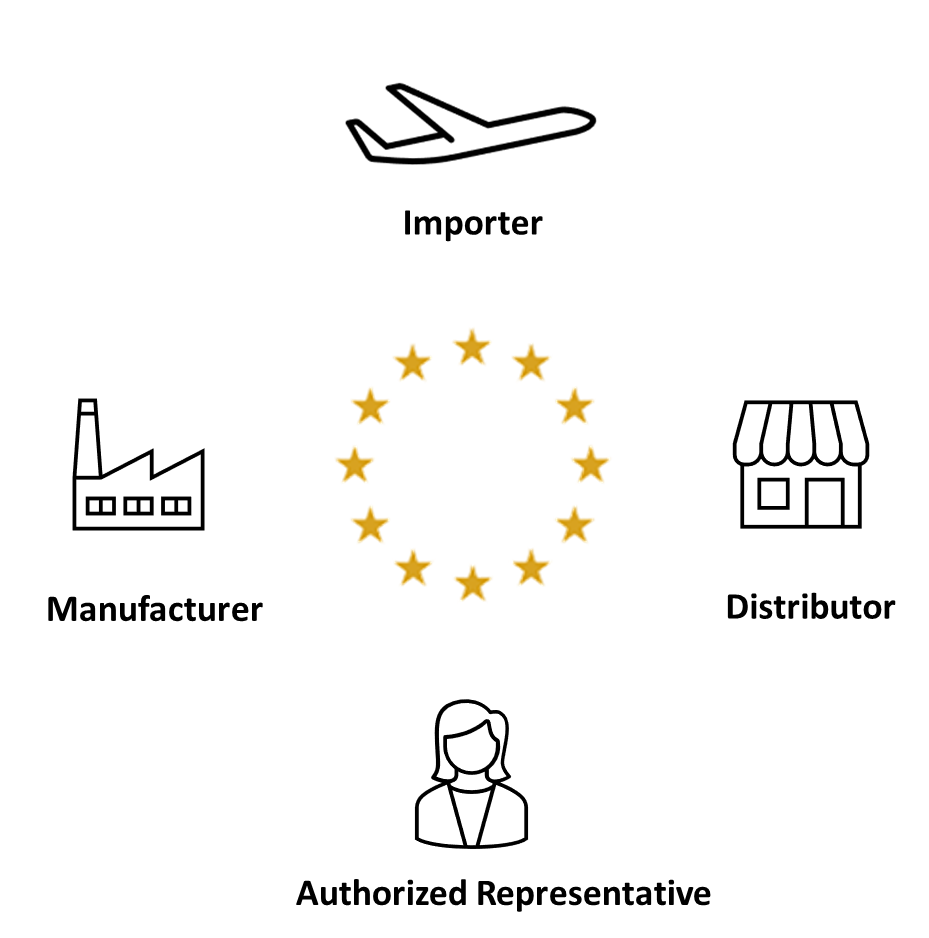
Medical Device Distributor Responsibilities . Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on.
from www.qualitiso.com
This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,.
Roles and obligations in the Medical Devices Regulation
Medical Device Distributor Responsibilities This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as.
From decomplix.com
Which MDR requirements apply to distributors of medical devices? Medical Device Distributor Responsibilities This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device. Medical Device Distributor Responsibilities.
From www.aeologic.com
Why is Traceability Essential for Medical Device Distributors? Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From flexlyconsulting.com
What Is GDPMD? Why Medical Device Distributor Needs It? Why Medical Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.lazada.com.ph
STANDARD OPERATING PROCEDURE AND RISK MANAGEMENT PLAN MEDICAL DEVICE Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.massoninternational.com
Compliance Guide Understanding Swiss Medical Device Distributors Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.slideserve.com
PPT Medical Device Distribution in Hong Kong_ A Vital Component of Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From www.qualitiso.com
Roles and obligations in the Medical Devices Regulation Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.aplyon.com
Medical Device Quality Agreement US Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From www.slideserve.com
PPT Medical Device Distribution in Hong Kong_ A Vital Component of Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical. Medical Device Distributor Responsibilities.
From www.slideserve.com
PPT Medical Device Reporting and Tracking PowerPoint Presentation Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.canada.ca
Guidance on Medical Device Establishment Licensing (GUI0016) Canada.ca Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products. Medical Device Distributor Responsibilities.
From www.sec.gov
Medical EquipmentMedical Equipment Distribution Government Procurement Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Medical. Medical Device Distributor Responsibilities.
From www.pinterest.com
100 CANSPAM, GDPR Compliant Medical Device Distributor Emails in 2023 Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical. Medical Device Distributor Responsibilities.
From shopee.ph
STANDARD OPERATING PROCEDURE AND RISK MANAGEMENT PLAN MEDICAL DEVICE Medical Device Distributor Responsibilities This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.slideserve.com
PPT Navigating the Future of Healthcare_ The Role of Medical Device Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From www.eurodev.com
Medical Device Distributorship Activating Your Distributors Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Medical. Medical Device Distributor Responsibilities.
From girp.eu
The role and value of fullservice healthcare distributors GIRP Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products. Medical Device Distributor Responsibilities.
From school.easymedicaldevice.com
Agreement Distributor Template Agreement (MDR & IVDR) Easy Medical Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From premier-research.com
7 Sponsor Responsibilities in Medical Device Clinical Trials Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From www.sec.gov
Medical EquipmentMedical Equipment Distribution Government Procurement Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.slidegeeks.com
Medical Device Distributor Opportunities Ppt PowerPoint Presentation Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.slideserve.com
PPT The Function of Medical Device Distributors in South Korea in Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From decomplix.com
Which MDR requirements apply to distributors of medical devices? Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products. Medical Device Distributor Responsibilities.
From www.healthcaretalentlink.com
How Medical Device Distributors Improve Patient Care HCTL Medical Device Distributor Responsibilities This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical. Medical Device Distributor Responsibilities.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare. Medical Device Distributor Responsibilities.
From www.aeologic.com
Why is Traceability Essential for Medical Device Distributors? Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical. Medical Device Distributor Responsibilities.
From starfishmedical.com
5 Top Annual Plan Medical Device Design and Development Process Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products. Medical Device Distributor Responsibilities.
From www.slideteam.net
Medical Device Distribution Channels In Powerpoint And Google Slides Cpb Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products. Medical Device Distributor Responsibilities.
From www.orielstat.com
European Medical Device Importers and Distributors Have New Regulatory Medical Device Distributor Responsibilities Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations,. Medical Device Distributor Responsibilities.
From www.greenlight.guru
How To Vet Medical Device Distributors in Multiple Markets Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device. Medical Device Distributor Responsibilities.
From cliniexperts.com
Medical Device Authorized Agent Role & Responsibilities CliniExperts Medical Device Distributor Responsibilities Medical device and ivd distributors endorse important duties relative to device conformity verification, device traceability,. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products. Medical Device Distributor Responsibilities.
From www.typecalendar.com
Free Printable Distribution Agreement Templates [Word, PDF] Simple Medical Device Distributor Responsibilities The regulations clarify the respective responsibilities of authorised representatives, importers and distributors, as. This article explains the role of a distributor under the new mdr and ivdr regulations, as well as reviewing the responsibilities of distributors in regards to placing products on. Distributors must prepare for new incoming requirements under eu medical device and in vitro diagnostic device regulations. Medical. Medical Device Distributor Responsibilities.