Aluminum Nitride Cation . Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. The oxygen atom has a 2− charge as an. This combination is written as \(\ce{alf3}\). Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Cations 1+ ammonium nh 4 + cesium. Here some of them are reviewed and. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Iron can form two possible ions, but the ion with a 3+ charge is specified here. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Write the formulas for aluminum nitride and lithium oxide.
from ijmmm.ustb.edu.cn
The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Cations 1+ ammonium nh 4 + cesium. Write the formulas for aluminum nitride and lithium oxide. This combination is written as \(\ce{alf3}\). The oxygen atom has a 2− charge as an. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Here some of them are reviewed and.
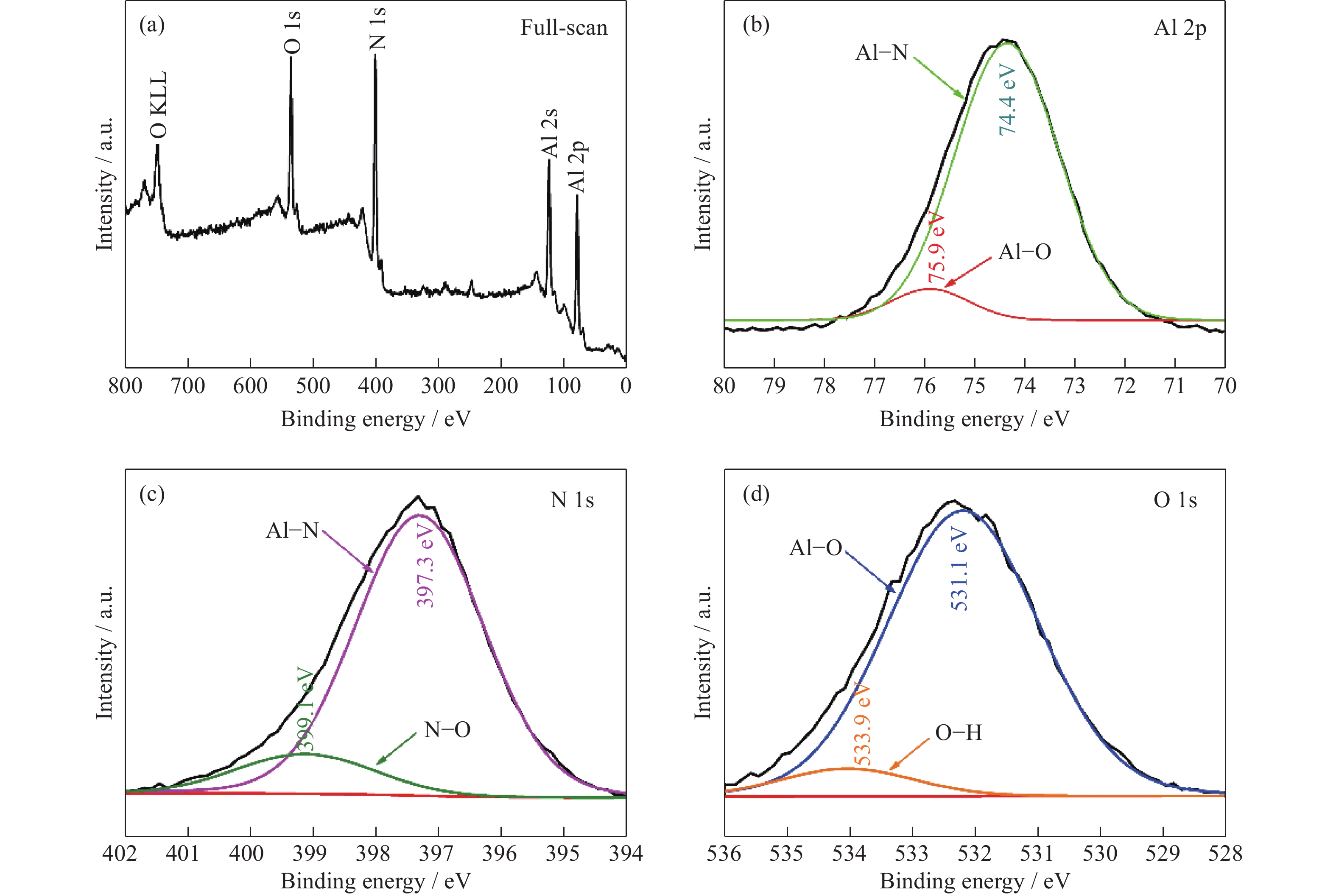
Morphology control of aluminum nitride (AlN) for a novel high
Aluminum Nitride Cation The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Write the formulas for aluminum nitride and lithium oxide. The oxygen atom has a 2− charge as an. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Cations 1+ ammonium nh 4 + cesium. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Here some of them are reviewed and. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. This combination is written as \(\ce{alf3}\).
From www.researchgate.net
(PDF) Aluminum Nitride in AlSiMg Alloy Aluminum Nitride Cation Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Three fluorine 1− ions are needed to balance the. Aluminum Nitride Cation.
From top-seiko.com
What is Aluminum Nitride (AlN) ? Top Seiko Co,. Ltd. Aluminum Nitride Cation The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Here some of them are reviewed and. This combination is written as \(\ce{alf3}\). Different from the oxidation kinetics of other nitrides, the oxide layer on aln can. Aluminum Nitride Cation.
From www.museoinclusivo.com
The Formula for Aluminum Nitride Understanding Its Composition and Aluminum Nitride Cation Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Write the formulas for aluminum nitride and lithium oxide. The oxygen atom has a 2− charge as an. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Three. Aluminum Nitride Cation.
From www.researchgate.net
Appearance of aluminum nitride powder before and after exposure to the Aluminum Nitride Cation Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Cations 1+ ammonium nh 4 + cesium. The lighter group 3a metals (aluminum, galium and. Aluminum Nitride Cation.
From www.youtube.com
TiAlN The titaniumaluminiumnitride coating YouTube Aluminum Nitride Cation Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. The oxygen atom has a 2− charge as an. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Write the formulas for aluminum nitride and lithium oxide. Cations 1+ ammonium nh 4 + cesium. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Aluminum Nitride Cation.
From slideplayer.com
Chapter 5 Online Resource ppt download Aluminum Nitride Cation This combination is written as \(\ce{alf3}\). Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. The oxygen atom has a 2− charge as an. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above.. Aluminum Nitride Cation.
From www.museoinclusivo.com
Exploring Aluminum Nitride Formula Uses, Advantages, and Innovative Aluminum Nitride Cation Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. This combination is written as \(\ce{alf3}\). Here some of them are reviewed and. The oxygen atom has a 2− charge as an. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a. Aluminum Nitride Cation.
From www.museoinclusivo.com
The Formula for Aluminum Nitride Understanding Its Composition and Aluminum Nitride Cation Cations 1+ ammonium nh 4 + cesium. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Three fluorine 1− ions are needed to balance the 3+ charge on. Aluminum Nitride Cation.
From www.preciseceramic.com
What is Aluminum Nitride Ceramic? Aluminum Nitride Cation Here some of them are reviewed and. Cations 1+ ammonium nh 4 + cesium. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. This combination is written as \(\ce{alf3}\). Different from the oxidation kinetics of other nitrides, the. Aluminum Nitride Cation.
From precision-ceramics.com
Aluminum Nitride CeramAlum™ Aluminum Nitride Cation Here some of them are reviewed and. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Cations 1+ ammonium nh 4 + cesium. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above.. Aluminum Nitride Cation.
From animalia-life.club
Cation And Anion Aluminum Nitride Cation Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Cations 1+ ammonium nh 4 + cesium. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Here some of them are reviewed and. Write the. Aluminum Nitride Cation.
From www.museoinclusivo.com
The Formula for Aluminum Nitride Understanding Its Composition and Aluminum Nitride Cation Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Nitride Cation.
From nanografi.com
Aluminum Nitride (AIN) Nanoparticles Nanografi Blog Nanografi Nano Aluminum Nitride Cation Here some of them are reviewed and. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Cations 1+ ammonium nh 4 + cesium. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. This. Aluminum Nitride Cation.
From www.chemistrylearner.com
Aluminum Nitride Facts, Formula, Properties, Uses, Toxicity Aluminum Nitride Cation Cations 1+ ammonium nh 4 + cesium. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Here some of them are reviewed and. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second.. Aluminum Nitride Cation.
From www.mdpi.com
Processes Free FullText Synthesis of Aluminum Nitride Using Sodium Aluminum Nitride Cation Here some of them are reviewed and. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. The oxygen atom has a 2− charge as an. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Iron can form two possible ions,. Aluminum Nitride Cation.
From ijmmm.ustb.edu.cn
Morphology control of aluminum nitride (AlN) for a novel high Aluminum Nitride Cation Cations 1+ ammonium nh 4 + cesium. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Here some of them are. Aluminum Nitride Cation.
From www.youtube.com
Chemistry 3Sec The detection of aluminum cation YouTube Aluminum Nitride Cation Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Cations 1+ ammonium nh 4 + cesium. Write the formulas for aluminum nitride and lithium oxide. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum. Aluminum Nitride Cation.
From www.researchgate.net
SEM of an Aluminum Nitride ceramic, densified with 2.5 wt La 2 O 3 Aluminum Nitride Cation This combination is written as \(\ce{alf3}\). Here some of them are reviewed and. Write the formulas for aluminum nitride and lithium oxide. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Different from the oxidation kinetics of other nitrides, the oxide. Aluminum Nitride Cation.
From pubs.acs.org
ScandiumDoped Aluminum Nitride for Acoustic Wave Resonators, Filters Aluminum Nitride Cation Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Here some of them are reviewed and. The oxygen atom has a 2− charge as an. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine. Aluminum Nitride Cation.
From www.cell.com
Removal of nitrides and fluorides from secondary aluminum dross by Aluminum Nitride Cation This combination is written as \(\ce{alf3}\). Iron can form two possible ions, but the ion with a 3+ charge is specified here. Here some of them are reviewed and. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Three fluorine 1− ions are needed to balance the 3+. Aluminum Nitride Cation.
From pubs.acs.org
Design of Multilayered Porous Aluminum Nitride for Supercapacitor Aluminum Nitride Cation Iron can form two possible ions, but the ion with a 3+ charge is specified here. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Three fluorine 1− ions are needed. Aluminum Nitride Cation.
From www.advaluetech.com
Aluminum Nitride Aluminum Nitride Cation Here some of them are reviewed and. Cations 1+ ammonium nh 4 + cesium. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. The oxygen atom has a 2− charge as an. Write the formulas for aluminum nitride and. Aluminum Nitride Cation.
From www.ledsmagazine.com
Aluminum nitridebased semiconductor by Tech unlocks Aluminum Nitride Cation Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Write the formulas for aluminum nitride and lithium oxide. Cations 1+ ammonium nh 4 + cesium. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine. Aluminum Nitride Cation.
From sciencetolf.weebly.com
Periodic table with charged ions sciencetolf Aluminum Nitride Cation Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. This combination is written as \(\ce{alf3}\). Write the formulas for aluminum nitride and lithium oxide. Cations 1+ ammonium nh 4 + cesium. Here some of them are reviewed and. The aluminum ion has a 3+ charge, while. Aluminum Nitride Cation.
From www.researchgate.net
a) Schematic band diagram at the surface of aluminum nitride (AlN). b Aluminum Nitride Cation Cations 1+ ammonium nh 4 + cesium. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Iron can form two possible ions, but the ion with a 3+ charge is specified here. This combination is written as \(\ce{alf3}\). Write the formulas for aluminum nitride and lithium oxide.. Aluminum Nitride Cation.
From www.researchgate.net
The crystal structures of (a) wurtzite phase of aluminum nitride as Aluminum Nitride Cation Cations 1+ ammonium nh 4 + cesium. Here some of them are reviewed and. The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Iron can form two possible ions, but the ion with a 3+ charge is specified here. The aluminum ion has a 3+ charge, while the. Aluminum Nitride Cation.
From www.youtube.com
How to Write the Formula for Aluminum nitride YouTube Aluminum Nitride Cation Write the formulas for aluminum nitride and lithium oxide. Cations 1+ ammonium nh 4 + cesium. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Iron can form two possible ions, but the ion with a. Aluminum Nitride Cation.
From www.numerade.com
SOLVEDCation Anion Chemical Formula Name Potassium nitride Aluminum Aluminum Nitride Cation The lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3] cations. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Here some of them are reviewed and. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Iron can. Aluminum Nitride Cation.
From slideplayer.com
Chemical Nomenclature ppt download Aluminum Nitride Cation The oxygen atom has a 2− charge as an. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Cations 1+ ammonium nh 4 + cesium. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Write the formulas for aluminum nitride. Aluminum Nitride Cation.
From www.nanotrun.com
What are Aluminum Nitride Characteristics? Aluminum Nitride Cation Iron can form two possible ions, but the ion with a 3+ charge is specified here. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Write the formulas for aluminum nitride and lithium oxide. Cations 1+ ammonium nh 4 + cesium. The oxygen atom has a 2− charge as an. Here some of them. Aluminum Nitride Cation.
From www.ck12.org
Ionic Compounds CK12 Foundation Aluminum Nitride Cation This combination is written as \(\ce{alf3}\). Iron can form two possible ions, but the ion with a 3+ charge is specified here. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. The oxygen atom has a 2− charge as an. Write the formulas for aluminum nitride. Aluminum Nitride Cation.
From www.mdpi.com
Processes Free FullText Synthesis of Aluminum Nitride Using Sodium Aluminum Nitride Cation Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. This combination is written as \(\ce{alf3}\). Here some of them are reviewed and. Cations 1+ ammonium nh 4 + cesium. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Write. Aluminum Nitride Cation.
From www.youtube.com
Nitride, Nitrite, and Nitrate Ions (Difference and Formulas) YouTube Aluminum Nitride Cation Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Here some of them are reviewed and. Aluminum al3+ chromium(iii) cr3+ cobalt(iii) co3+ gold. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Write the formulas for aluminum nitride and lithium oxide. Iron can form two possible ions, but. Aluminum Nitride Cation.
From www.innovacera.com
Aluminum Nitride Properties And Applications INNOVACERA Aluminum Nitride Cation Cations 1+ ammonium nh 4 + cesium. This combination is written as \(\ce{alf3}\). Iron can form two possible ions, but the ion with a 3+ charge is specified here. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Three fluorine 1− ions are needed to balance. Aluminum Nitride Cation.
From pubs.rsc.org
Boronnitride and aluminumnitride “Pringles” and flapping motion Aluminum Nitride Cation Cations 1+ ammonium nh 4 + cesium. Here some of them are reviewed and. This combination is written as \(\ce{alf3}\). Write the formulas for aluminum nitride and lithium oxide. Different from the oxidation kinetics of other nitrides, the oxide layer on aln can easily reach tens of micrometers at a temperature above. Iron can form two possible ions, but the. Aluminum Nitride Cation.