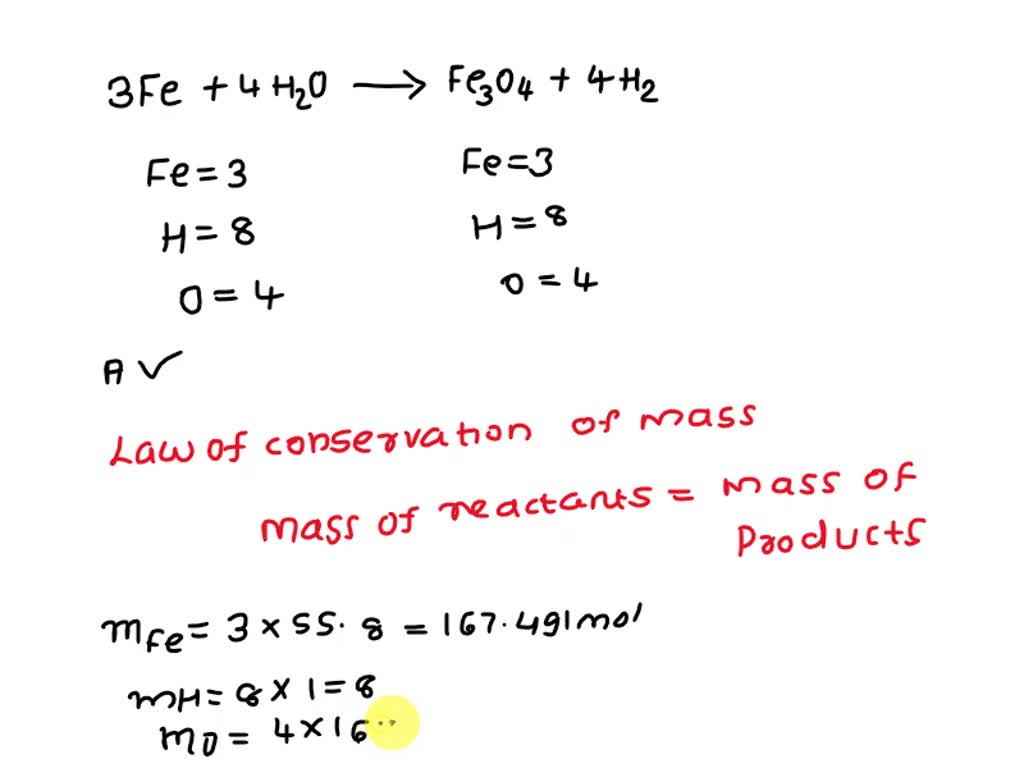Iron + Steam Equation . Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. Write a balanced equation for the reactions that occur when: Now, we can write the balanced equation for the reaction between iron. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Steam is water in its gaseous state, which has the chemical formula h2o. The reduction of water by iron stops at $\ce{fe3o4}$. Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Above $\pu{900 k}$ , it stops even at $\ce{feo}$. (i) iron react with steam. Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. This video explains how iron reacts with steam.our website: (ii) calcium and potassium react with water.
from www.numerade.com
Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Steam is water in its gaseous state, which has the chemical formula h2o. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Write a balanced equation for the reactions that occur when: Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. This video explains how iron reacts with steam.our website: Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. (ii) calcium and potassium react with water.
Assertion Following is a balanced chemical equation for the action of
Iron + Steam Equation Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. (i) iron react with steam. Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Now, we can write the balanced equation for the reaction between iron. Steam is water in its gaseous state, which has the chemical formula h2o. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. (ii) calcium and potassium react with water. This video explains how iron reacts with steam.our website: Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. Write a balanced equation for the reactions that occur when: The reduction of water by iron stops at $\ce{fe3o4}$.
From www.youtube.com
Steam Equations in Basic Mechanical Engineering, SteamEnthalpy, Iron + Steam Equation Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. This video explains how iron reacts with steam.our website: Iron has three different. Iron + Steam Equation.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Iron + Steam Equation Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Steam is water in its gaseous state, which has the chemical formula h2o. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. (i) iron react with steam. This video explains how iron reacts with steam.our website: Write a balanced equation for the reactions that occur when: (ii) calcium and potassium react. Iron + Steam Equation.
From www.teachoo.com
Write equations for reaction of (i) iron with steam Class 10 Science Iron + Steam Equation Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. The reduction of water by iron stops at $\ce{fe3o4}$. Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. Recap the reactivity. Iron + Steam Equation.
From signalticket9.pythonanywhere.com
Spectacular Iron Oxide State Symbol Www Physics Wallah Com Notes Pdf Iron + Steam Equation (i) iron react with steam. Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. Now, we can write the balanced equation for the reaction between iron. (ii) calcium and potassium react with water. This video explains how iron reacts with steam.our website: Iron has three different oxides, and. Iron + Steam Equation.
From www.chegg.com
Solved x for a particular steam iron are related by the Iron + Steam Equation Now, we can write the balanced equation for the reaction between iron. This video explains how iron reacts with steam.our website: Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Write a balanced equation. Iron + Steam Equation.
From brainly.in
Assertion Following is a balanced chemical equation for the action of Iron + Steam Equation (i) iron react with steam. Write a balanced equation for the reactions that occur when: Now, we can write the balanced equation for the reaction between iron. (ii) calcium and potassium react with water. Steam is water in its gaseous state, which has the chemical formula h2o. This video explains how iron reacts with steam.our website: Above $\pu{900 k}$ ,. Iron + Steam Equation.
From mammothmemory.net
Iron introduced to heat and steam turns to black iron oxide Iron + Steam Equation Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Now, we can write the balanced equation for the. Iron + Steam Equation.
From www.numerade.com
Assertion Following is a balanced chemical equation for the action of Iron + Steam Equation Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. Write a balanced equation for the reactions that occur when: Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Now, we can write the balanced equation for the reaction between. Iron + Steam Equation.
From www.doubtnut.com
Write equations for the reactions. (i) Iron with steam (ii) Calciu Iron + Steam Equation Write a balanced equation for the reactions that occur when: The reduction of water by iron stops at $\ce{fe3o4}$. Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned. Iron + Steam Equation.
From www.toppr.com
a) Write the equations for the following and balance them Iron reacts Iron + Steam Equation (i) iron react with steam. Write a balanced equation for the reactions that occur when: Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. (ii) calcium and potassium react with water. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Why is the product of the reaction between. Iron + Steam Equation.
From www.youtube.com
chemical equation for reaction of iron with steam, calcium with water Iron + Steam Equation This video explains how iron reacts with steam.our website: Above $\pu{900 k}$ , it stops even at $\ce{feo}$. (ii) calcium and potassium react with water. Write a balanced equation for the reactions that occur when: Now, we can write the balanced equation for the reaction between iron. Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Iron has three different. Iron + Steam Equation.
From www.theopendictionary.com
Steam iron Meaning of Steam iron Definition of Steam iron Example Iron + Steam Equation This video explains how iron reacts with steam.our website: (i) iron react with steam. Write a balanced equation for the reactions that occur when: Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Write balanced equations for (a) the reaction of iron with steam,. Iron + Steam Equation.
From www.toppr.com
4. 3M Write balanced equations the reactions of i) Aluminium when Iron + Steam Equation (ii) calcium and potassium react with water. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. This video explains how iron reacts with steam.our website: Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. The reduction of water by. Iron + Steam Equation.
From www.youtube.com
Reaction of metals with steam YouTube Iron + Steam Equation Now, we can write the balanced equation for the reaction between iron. This video explains how iron reacts with steam.our website: (i) iron react with steam. Write a balanced equation for the reactions that occur when: (ii) calcium and potassium react with water. The reduction of water by iron stops at $\ce{fe3o4}$. Steam is water in its gaseous state, which. Iron + Steam Equation.
From www.numerade.com
SOLVEDwrite the chemical equation for the reaction was description is Iron + Steam Equation Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide,. Iron + Steam Equation.
From bestofiron.com
Dry Iron Vs. Steam Iron. Which is better? Iron + Steam Equation Steam is water in its gaseous state, which has the chemical formula h2o. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. (ii) calcium and potassium react with water. Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Iron has three different oxides,. Iron + Steam Equation.
From askfilo.com
(2.) Write equations for the reactions of (i) iron with steam (ii) calciu.. Iron + Steam Equation (i) iron react with steam. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. (ii) calcium and potassium react with water. Now, we can write the balanced equation for the reaction between iron. This. Iron + Steam Equation.
From www.slideshare.net
Chemical Reactions Iron + Steam Equation (ii) calcium and potassium react with water. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Write a balanced equation for the reactions that occur when: Steam is water in its gaseous state, which has the chemical formula h2o. (i) iron react with. Iron + Steam Equation.
From www.youtube.com
HOW TO USE STEAM IRONSTEAM IRON YouTube Iron + Steam Equation Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. The reduction of water by iron stops at $\ce{fe3o4}$. Now, we can write the balanced equation for the reaction between iron. This video explains how iron reacts with steam.our website:. Iron + Steam Equation.
From www.youtube.com
Write equations for the reactions of (i) iron with steam (ii) calcium Iron + Steam Equation Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Now, we can write the. Iron + Steam Equation.
From brainly.in
Write balanced chemical equations for the reactions of (i) iron with Iron + Steam Equation Write a balanced equation for the reactions that occur when: The reduction of water by iron stops at $\ce{fe3o4}$. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Steam is water in its gaseous state, which has the chemical formula h2o. This video explains. Iron + Steam Equation.
From www.slideshare.net
Metals Iron + Steam Equation Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Write a balanced equation for the reactions that occur when: (i) iron react with steam. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and. Iron + Steam Equation.
From brainly.in
Write an equation for the following reaction. Iron reacts with steam Iron + Steam Equation Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. (ii) calcium and potassium react with water. This video explains how iron. Iron + Steam Equation.
From www.slideserve.com
PPT Tips To Use A Steam Iron PowerPoint Presentation, free download Iron + Steam Equation Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Iron has three different oxides, and the one formed. Iron + Steam Equation.
From www.youtube.com
iGCSE / GCSE Chemistry Extraction of iron (10.1) YouTube Iron + Steam Equation Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. The reduction of water by iron stops at $\ce{fe3o4}$. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide. Iron + Steam Equation.
From www.youtube.com
Iron + Water = Rust Chemistry! Balancing Equations, Coefficients Iron + Steam Equation The reduction of water by iron stops at $\ce{fe3o4}$. (ii) calcium and potassium react with water. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Steam is water in its gaseous state, which has the chemical formula h2o. Write a balanced equation for the. Iron + Steam Equation.
From brainly.in
Write equations for the reactions of (i) iron with steam (ii) calcium Iron + Steam Equation (ii) calcium and potassium react with water. Steam is water in its gaseous state, which has the chemical formula h2o. (i) iron react with steam. Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. Recap the reactivity series of. Iron + Steam Equation.
From brainly.in
Iron reacts with steam to produce triferric tetraoxide and hydrogen Iron + Steam Equation Now, we can write the balanced equation for the reaction between iron. This video explains how iron reacts with steam.our website: Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. (ii) calcium and potassium react with water. Iron has three different oxides, and the one formed when you. Iron + Steam Equation.
From www.youtube.com
REACTION OF IRON WITH STEAM YouTube Iron + Steam Equation This video explains how iron reacts with steam.our website: Write a balanced equation for the reactions that occur when: Steam is water in its gaseous state, which has the chemical formula h2o. Recap the reactivity series of metals and how it's used to predict the outcomes of reactions with air, water and steam as well as extracting aluminium. Above $\pu{900. Iron + Steam Equation.
From brainly.in
reaction of iron with steam Brainly.in Iron + Steam Equation Above $\pu{900 k}$ , it stops even at $\ce{feo}$. The reduction of water by iron stops at $\ce{fe3o4}$. Now, we can write the balanced equation for the reaction between iron. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Iron has three different oxides,. Iron + Steam Equation.
From www.numerade.com
SOLVEDWrite balanced equations for (a) the reaction of iron with steam Iron + Steam Equation (i) iron react with steam. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Now, we can write the balanced equation for the reaction between iron. This video explains how iron reacts with steam.our website:. Iron + Steam Equation.
From www.youtube.com
Diagram Of Action Of Steam On A Metal Diagram Class 10 Iron + Steam Equation Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. (ii) calcium and potassium react with water. Above $\pu{900 k}$ , it stops even at $\ce{feo}$. Now, we can write the balanced equation for the reaction between iron. The reduction of water by iron stops at $\ce{fe3o4}$. This video. Iron + Steam Equation.
From askfilo.com
5. Write balanced equations for the reaction of a. Iron with steam. Name.. Iron + Steam Equation Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o 4. The reduction of water by iron stops at $\ce{fe3o4}$. (i) iron react with steam. Steam is water in its gaseous state, which has the chemical formula h2o. Recap the reactivity. Iron + Steam Equation.
From www.teachoo.com
Chemical Properties of Metals [with Reaction Examples] Teachoo Iron + Steam Equation (ii) calcium and potassium react with water. Steam is water in its gaseous state, which has the chemical formula h2o. Above $\pu{1700 k}$ , hydrogen reduces $\ce{feo}$ to iron. Why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Iron has three different oxides, and. Iron + Steam Equation.
From www.youtube.com
Write equations for the reactions of(i) iron with steam (ii) calcium Iron + Steam Equation Write a balanced equation for the reactions that occur when: Steam is water in its gaseous state, which has the chemical formula h2o. (i) iron react with steam. (ii) calcium and potassium react with water. Write balanced equations for (a) the reaction of iron with steam, (b) the reaction of calcium with hydrochloric acid, (c) the. Now, we can write. Iron + Steam Equation.