Is Liquid Included In Equilibrium Constant . — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. They are, in a way. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. [op] why are solids and liquids not included in the equilibrium constant? the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. Writing an expression for kc. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium.
from present5.com
in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. Writing an expression for kc. [op] why are solids and liquids not included in the equilibrium constant? — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. They are, in a way.
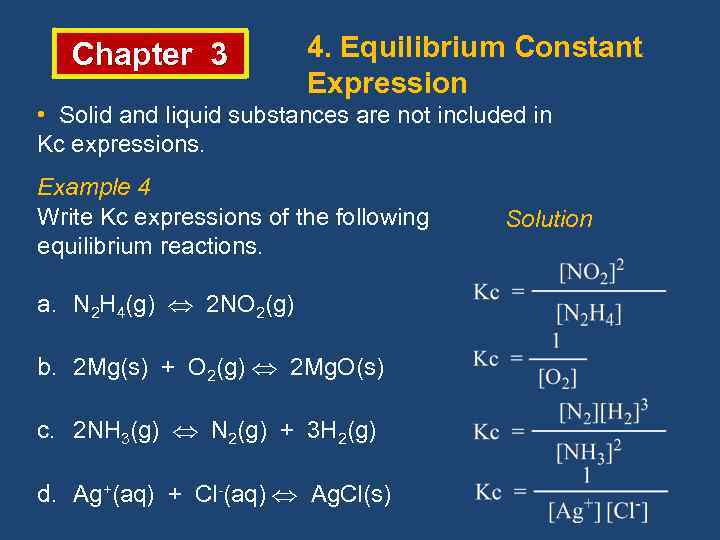
Chapter 3 Chemical Equilibrium Reactions Irreversible Reversible The
Is Liquid Included In Equilibrium Constant the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. They are, in a way. [op] why are solids and liquids not included in the equilibrium constant? — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Writing an expression for kc. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the.
From www.youtube.com
Equilibrium Constant (Ideal Gases) YouTube Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. They are, in a way. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. Writing an. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
15.5 Heterogeneous Equilibrium Equilibrium Expression Involving a Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. Writing an expression for kc. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. in. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Section 8.2—Equilibrium Constant PowerPoint Presentation, free Is Liquid Included In Equilibrium Constant in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. — the equilibrium constant, k, expresses the relationship. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Is Liquid Included In Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? They are, in a way. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. — when dealing with. Is Liquid Included In Equilibrium Constant.
From www.pinterest.co.uk
Law of Chemical Equilibrium, Equilibrium Constant Examples Is Liquid Included In Equilibrium Constant — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. [op] why are solids and liquids not included in the equilibrium. Is Liquid Included In Equilibrium Constant.
From www.numerade.com
SOLVED\( \mathrm{} \) \( \mathrm{} \) Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. Writing an expression for kc. the subscript p in. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Equilibrium Expressions PowerPoint Presentation, free download Is Liquid Included In Equilibrium Constant — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. They are, in. Is Liquid Included In Equilibrium Constant.
From thechemistrynotes.com
Equilibrium Constant Definition, Characteristics, and Calculations Is Liquid Included In Equilibrium Constant Writing an expression for kc. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. [op] why are solids and liquids not included in the equilibrium constant? They are, in a way. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
How to Calculate Equilibrium Constant Keq // HSC Chemistry YouTube Is Liquid Included In Equilibrium Constant Writing an expression for kc. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. . Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is Liquid Included In Equilibrium Constant the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. They are, in a way. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. Writing an expression for kc. [op] why are solids and liquids not included in the equilibrium constant? . Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT 16 Chemical Equilibrium PowerPoint Presentation, free download Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. [op] why are solids and liquids not included in the equilibrium constant? — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. Writing an expression for kc. in equations 6 through. Is Liquid Included In Equilibrium Constant.
From www.askiitians.com
The equilibrium constant for the reaction CO(g)+ H2O(g) =CO2 (g)+ H2 Is Liquid Included In Equilibrium Constant They are, in a way. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Chapter 13 Chemical Equilibrium PowerPoint Presentation, free Is Liquid Included In Equilibrium Constant — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. [op] why are solids and liquids not included in the equilibrium constant? — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the subscript p in the symbol k p designates an equilibrium constant. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
EQUILIBRIUM CONSTANT AND UNIT PART 1 / CONTEXT OF CHEMISTRY YouTube Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Writing an expression for kc. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. They are, in a way.. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
The equilibrium constant Kp YouTube Is Liquid Included In Equilibrium Constant — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. Writing an expression for kc. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. — because equilibrium can be approached. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is Liquid Included In Equilibrium Constant — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. Writing an expression for kc. They are, in a way. [op] why are solids and liquids not included in the equilibrium constant? the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
Understanding The Equilibrium Constant, Kp (A2 Chemistry) YouTube Is Liquid Included In Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. in equations 6 through 10, you saw how the constant concentration of. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Calculating Equilibrium Constants PowerPoint Presentation, free Is Liquid Included In Equilibrium Constant Writing an expression for kc. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. They are, in a way.. Is Liquid Included In Equilibrium Constant.
From www.coursehero.com
[Solved] . Express the equilibrium constant for the following reaction Is Liquid Included In Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. in equations 6 through 10, you saw. Is Liquid Included In Equilibrium Constant.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, An Introduction Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. [op] why are solids and liquids not included in the equilibrium constant? Writing an expression for kc. in equations 6. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free Is Liquid Included In Equilibrium Constant in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. They are, in a way. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — the. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
Properties of the equilibrium constant Equilibrium AP Chemistry Is Liquid Included In Equilibrium Constant Writing an expression for kc. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. . Is Liquid Included In Equilibrium Constant.
From www.physicsforums.com
Solving Equilibrium Constant Is Liquid Included In Equilibrium Constant — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. the subscript p in the symbol k p designates an equilibrium. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
The equilibrium expression and the equilibrium constant (Keq) YouTube Is Liquid Included In Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Writing an expression for kc. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — because equilibrium. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
Explain why pure liquids and solids can be ignored while writing the Is Liquid Included In Equilibrium Constant Writing an expression for kc. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. They are, in a way. in equations 6 through 10, you saw how the constant concentration. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is Liquid Included In Equilibrium Constant — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. They are, in a way. Writing an expression for kc. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. — the equilibrium constant, k, expresses the relationship between products and reactants of a. Is Liquid Included In Equilibrium Constant.
From secondaryscience4all.wordpress.com
1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2 Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. Writing an expression for kc. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead. Is Liquid Included In Equilibrium Constant.
From present5.com
Chapter 3 Chemical Equilibrium Reactions Irreversible Reversible The Is Liquid Included In Equilibrium Constant [op] why are solids and liquids not included in the equilibrium constant? — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. the subscript p in the symbol k p designates an equilibrium. Is Liquid Included In Equilibrium Constant.
From studylib.net
Equilibrium for a general reaction Is Liquid Included In Equilibrium Constant in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. Writing an expression for kc. [op] why are solids and liquids not included in the equilibrium constant? — because equilibrium can be approached from either direction. Is Liquid Included In Equilibrium Constant.
From studylib.net
The equilibrium constant, K Is Liquid Included In Equilibrium Constant in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. [op] why are solids and liquids not included in the equilibrium constant? Writing an expression for kc. They are, in a way. — when dealing with. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Is Liquid Included In Equilibrium Constant They are, in a way. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. in equations 6 through 10, you saw how the constant concentration of a solid (and the. Is Liquid Included In Equilibrium Constant.
From www.youtube.com
The dissociation of water reaction, & the equilibrium constant, Kw Is Liquid Included In Equilibrium Constant in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant expression and. — the equilibrium constant, k, expresses the relationship. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Unit 9 and Equilibrium PowerPoint Presentation, free Is Liquid Included In Equilibrium Constant — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. They are, in a way. the subscript p in the symbol k p designates an equilibrium constant derived using partial pressures instead of concentrations. — because equilibrium can be approached from either direction in a chemical reaction, the equilibrium constant. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Is Liquid Included In Equilibrium Constant Writing an expression for kc. — when dealing with partial pressures, \(k_p\) is used, whereas when dealing with concentrations (molarity),. in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. — because equilibrium can be approached. Is Liquid Included In Equilibrium Constant.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Is Liquid Included In Equilibrium Constant in equations 6 through 10, you saw how the constant concentration of a solid (and the same argument would hold for a liquid) can be used to exclude it from the. — the equilibrium constant, k, expresses the relationship between products and reactants of a reaction at equilibrium. the subscript p in the symbol k p designates. Is Liquid Included In Equilibrium Constant.