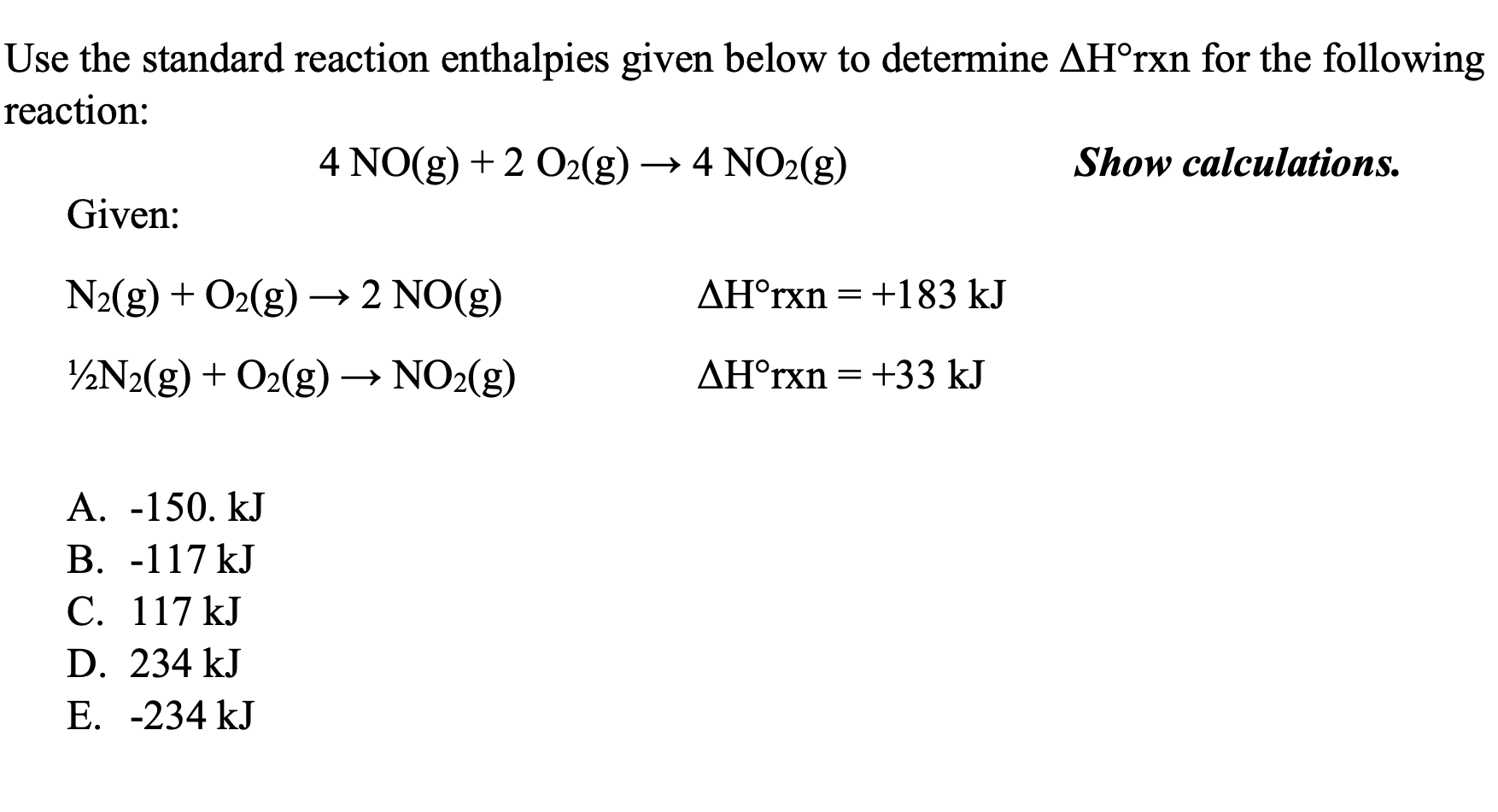
Use Standard Enthalpies Of Formation To Calculate For Each Reaction . Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Cao(s) + co 2 (g) → caco 3 (s)
from pixmob.info
standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: Cao(s) + co 2 (g) → caco 3 (s)
Use The Standard Reaction Enthalpies Given Below PIXMOB
Use Standard Enthalpies Of Formation To Calculate For Each Reaction a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Cao(s) + co 2 (g) → caco 3 (s) the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation:
From pixmob.info
Use The Standard Reaction Enthalpies Given Below PIXMOB Use Standard Enthalpies Of Formation To Calculate For Each Reaction because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Use Standard Enthalpies Of Formation To Calculate For Each Reaction Cao(s) + co 2 (g) → caco 3 (s) standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Use standard enthalpies of formation to find the. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Use Standard Enthalpies Of Formation To Calculate For Each Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. standard enthalpies of. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.gauthmath.com
Solved Use standard enthalpies of formation ( H values) to calculate Use Standard Enthalpies Of Formation To Calculate For Each Reaction standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: Cao(s) + co 2 (g) → caco 3 (s) 193 rows in chemistry and thermodynamics, the standard enthalpy of. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Use standard enthalpies of formation from the Use Standard Enthalpies Of Formation To Calculate For Each Reaction Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as Cao(s) + co 2 (g) → caco 3 (s) because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVEDUse the standard enthalpies of formation available here to Use Standard Enthalpies Of Formation To Calculate For Each Reaction Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. the standard enthalpy of. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate ΔH° Use Standard Enthalpies Of Formation To Calculate For Each Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as a standard enthalpy of formation $δh _f$ is an enthalpy change. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Use Standard Enthalpies Of Formation To Calculate For Each Reaction a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation To Calculate For Each Reaction because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.coursehero.com
[Solved] First, balance the reaction and then use the standard Use Standard Enthalpies Of Formation To Calculate For Each Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Ch 4. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVED Use standard enthalpies of formation (AH; values) to calculate Use Standard Enthalpies Of Formation To Calculate For Each Reaction Cao(s) + co 2 (g) → caco 3 (s) 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l). Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Calculate the enthalpy of the following reaction Use Standard Enthalpies Of Formation To Calculate For Each Reaction because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. standard enthalpies of formation the standard molar enthalpy of formation δh o f is. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Part A Use standard enthalpies of formation to Use Standard Enthalpies Of Formation To Calculate For Each Reaction standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.transtutors.com
(Get Answer) use the standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation To Calculate For Each Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as standard enthalpies of formation the standard molar enthalpy of formation δh. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVED Calculate values for the standard reaction enthalpies at 298k Use Standard Enthalpies Of Formation To Calculate For Each Reaction use standard enthalpies of formation to calculate δh∘rxn for the following reaction: because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: Ch 4 (g) + 2 o 2 (g) →. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVEDUse enthalpies of formation below to calculate the standard Use Standard Enthalpies Of Formation To Calculate For Each Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: 193 rows in chemistry. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Use Standard Enthalpies Of Formation To Calculate For Each Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. Cao(s) + co 2 (g) → caco 3 (s) because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. 193 rows in chemistry and thermodynamics, the standard enthalpy. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Use Standard Enthalpies Of Formation To Calculate For Each Reaction use standard enthalpies of formation to calculate δh∘rxn for the following reaction: Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. 193 rows in chemistry and thermodynamics,. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation To Calculate For Each Reaction Cao(s) + co 2 (g) → caco 3 (s) because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Use Standard Enthalpies Of Formation To Calculate For Each Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Use Standard Enthalpies Of Formation To Calculate For Each Reaction because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as the standard enthalpy of reaction (\(δh^o_{rxn}\)). Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Use Standard Enthalpies Of Formation To Calculate For Each Reaction a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: Cao(s) + co 2 (g) → caco 3 (s) 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVED Using the standard molar enthalpies of formation given Use Standard Enthalpies Of Formation To Calculate For Each Reaction because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. Cao(s) + co 2 (g) → caco 3 (s) use standard enthalpies of formation to calculate δh∘rxn for the following reaction: Use standard enthalpies of formation to find the enthalpy of reaction for the following equation:. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.answersarena.com
[Solved] 4. Use the standard enthalpies of formation t Use Standard Enthalpies Of Formation To Calculate For Each Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. because there is one mole each of a, b and c, the standard enthalpy of formation. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVED Use standard enthalpies of formation to calculate the standard Use Standard Enthalpies Of Formation To Calculate For Each Reaction Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. Cao(s) +. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVEDUse standard enthalpies of formation from Table 7.2 to determine Use Standard Enthalpies Of Formation To Calculate For Each Reaction Cao(s) + co 2 (g) → caco 3 (s) the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation To Calculate For Each Reaction the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. Cao(s) + co 2 (g) → caco 3 (s) because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. Ch 4 (g) + 2 o 2 (g) → co. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.solutionspile.com
[Solved] Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation To Calculate For Each Reaction Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as because there is one mole each of a, b and c, the standard enthalpy of formation of each reactant and product is. Cao(s) + co 2 (g) → caco. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate A, Use Standard Enthalpies Of Formation To Calculate For Each Reaction Cao(s) + co 2 (g) → caco 3 (s) Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.bartleby.com
Answered Use the standard reaction enthalpies… bartleby Use Standard Enthalpies Of Formation To Calculate For Each Reaction Ch 4 (g) + 2 o 2 (g) → co 2 (g) + 2 h 2 o (l) solution from the above table, the standard enthalpies of formation are as a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Use standard enthalpies of formation to find the. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From tutorbin.com
Solved Use enthalpies of formation to calculate the standard enthalpy Use Standard Enthalpies Of Formation To Calculate For Each Reaction a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. 193 rows in chemistry. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.youtube.com
Calculating Reaction Enthalpy from Enthalpies of Formation YouTube Use Standard Enthalpies Of Formation To Calculate For Each Reaction Use standard enthalpies of formation to find the enthalpy of reaction for the following equation: a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. use standard enthalpies of formation to calculate δh∘rxn for the following reaction: because there is one mole each of a, b. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.bartleby.com
Answered Use standard enthalpies of formation to… bartleby Use Standard Enthalpies Of Formation To Calculate For Each Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Cao(s) + co 2 (g) → caco 3 (s) the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. Use standard enthalpies of formation to find the enthalpy of reaction for the following equation:. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVED Use the standard enthalpies of formation in the table below to Use Standard Enthalpies Of Formation To Calculate For Each Reaction use standard enthalpies of formation to calculate δh∘rxn for the following reaction: the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of. standard enthalpies of formation the standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance,. Cao(s) + co 2. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.
From www.numerade.com
SOLVEDUsing enthalpies of formation (Appendix C), calculate ΔH^∘ for Use Standard Enthalpies Of Formation To Calculate For Each Reaction 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh _f$ is an enthalpy change for a reaction in which exactly 1 mole of a. Cao(s) + co 2 (g) → caco 3 (s) because there is one mole each of a, b and. Use Standard Enthalpies Of Formation To Calculate For Each Reaction.