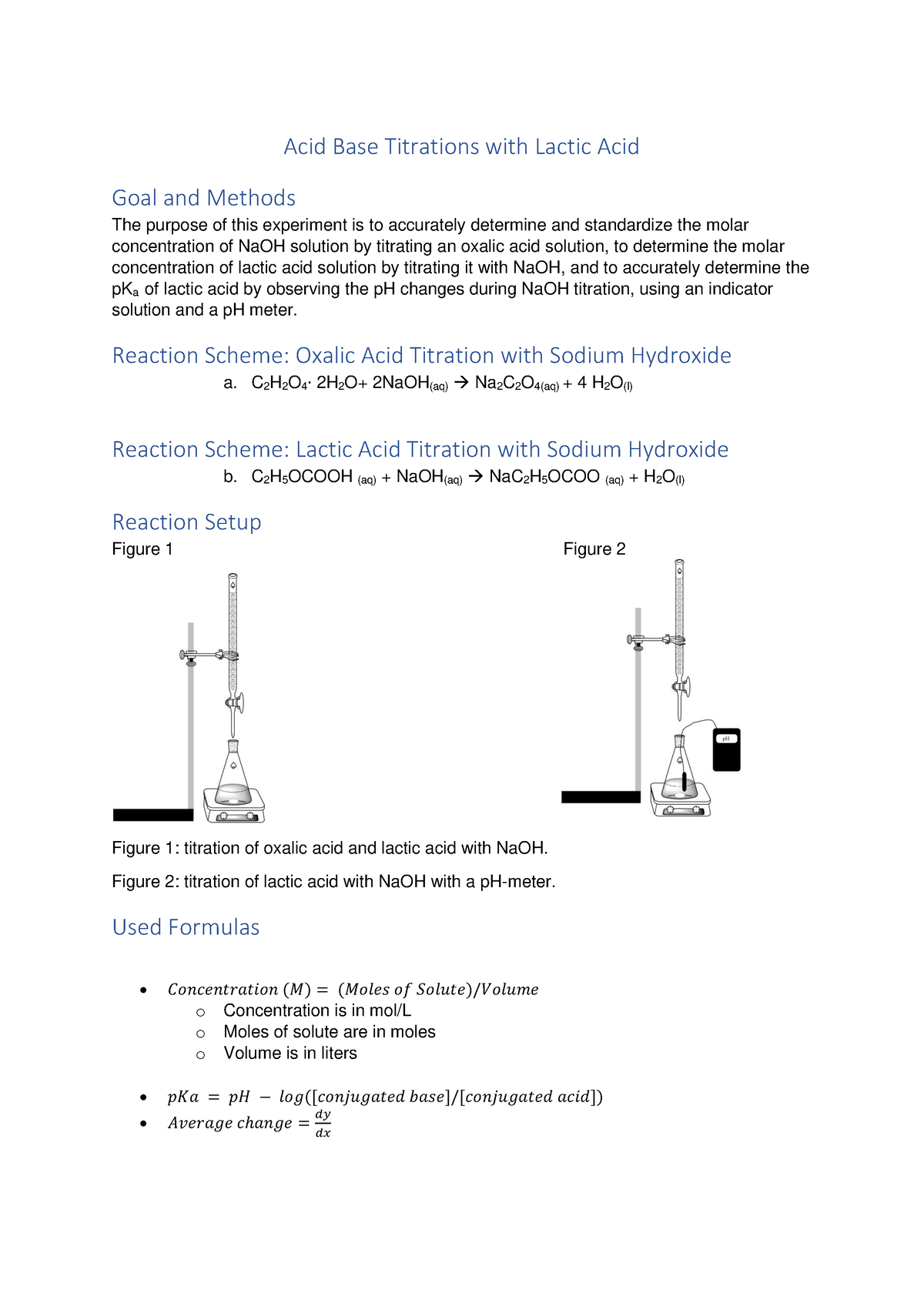
Lab Acid Base Titration . The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is an analytical quantitative technique used to determine the concentration of a solute; Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Includes kit list and safety instructions. Titrations play an important role in determining amount and purity in many manufacturing processes.
from www.studeersnel.nl
The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is a common laboratory technique for determining the concentration of a solute. Titrations play an important role in determining amount and purity in many manufacturing processes. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions.
Acid Base Lab lactic acid titration lab report Acid Base Titrations
Lab Acid Base Titration Titration is an analytical quantitative technique used to determine the concentration of a solute; Titration is an analytical quantitative technique used to determine the concentration of a solute; The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is a common laboratory technique for determining the concentration of a solute. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Titrations play an important role in determining amount and purity in many manufacturing processes.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Lab Acid Base Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titrations play an important. Lab Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab Acid Base Titration Includes kit list and safety instructions. Titrations play an important role in determining amount and purity in many manufacturing processes. Titration is an analytical quantitative technique used to determine the concentration of a solute; It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Use this class practical to explore. Lab Acid Base Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titrations play an important role in determining amount and purity in many manufacturing processes. Titration is a common laboratory technique for determining the concentration of a solute. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric. Lab Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab Acid Base Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titrations play an important role in determining amount and purity in many manufacturing processes. It is based on the neutralization. Lab Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Procedure Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titrations play an important role in determining amount and purity in many manufacturing processes. Titration is a common laboratory technique for determining the concentration of a solute. Be able to determine the k a or k b from ph data associated with the titration. Lab Acid Base Titration.
From www.sliderbase.com
Titration Lab Acid Base Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. Titration is an analytical quantitative technique used. Lab Acid Base Titration.
From mavink.com
Acid Base Titration Lab Procedure Lab Acid Base Titration Titration is a common laboratory technique for determining the concentration of a solute. Titration is an analytical quantitative technique used to determine the concentration of a solute; Includes kit list and safety instructions. Titrations play an important role in determining amount and purity in many manufacturing processes. Use this class practical to explore titration, producing the salt sodium chloride with. Lab Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. Titration is an analytical quantitative technique used to determine. Lab Acid Base Titration.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Lab Acid Base Titration Titration is a common laboratory technique for determining the concentration of a solute. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Be able to determine the k a or k b from. Lab Acid Base Titration.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is an analytical quantitative technique used to determine the concentration of a solute; Titration is a common laboratory technique for determining the concentration of a solute. It is based on the neutralization reaction, where an acid and a base react to form water. Lab Acid Base Titration.
From joixjswcw.blob.core.windows.net
Titration Of Acid Base Experiment at Joanne Rivera blog Lab Acid Base Titration Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. Titrations play an important role in determining amount and purity in many manufacturing processes. The concentration of a solution can be determined by knowing the acid and base dissociation. Lab Acid Base Titration.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Lab Acid Base Titration Titration is an analytical quantitative technique used to determine the concentration of a solute; Titration is a common laboratory technique for determining the concentration of a solute. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titrations play an important role in determining amount and purity in many manufacturing processes. Use this class. Lab Acid Base Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is an analytical quantitative technique used to determine the concentration of a solute; Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. It is based on the neutralization reaction, where an acid and a. Lab Acid Base Titration.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Lab Acid Base Titration Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Includes kit list and safety instructions. It is based on the neutralization reaction, where an acid and a base react to form water and. Lab Acid Base Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Lab Acid Base Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is an analytical quantitative technique used to determine the concentration of a solute; Titration is a common laboratory technique for determining the concentration of a solute. Includes kit list and safety instructions. The concentration of a solution. Lab Acid Base Titration.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Lab Acid Base Titration Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titrations play an important role in determining amount and purity in many manufacturing processes. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration is an analytical quantitative technique. Lab Acid Base Titration.
From www.slideshare.net
Acid base titration (1) Lab Acid Base Titration Titrations play an important role in determining amount and purity in many manufacturing processes. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. It is based on the neutralization. Lab Acid Base Titration.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Lab Acid Base Titration Titrations play an important role in determining amount and purity in many manufacturing processes. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated. Lab Acid Base Titration.
From www.visionlearning.com
Ácidos y Bases I Chemistry Visionlearning Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is an analytical quantitative technique used to determine the concentration of a solute; It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titrations play an important role in determining amount and purity. Lab Acid Base Titration.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Lab Acid Base Titration Titrations play an important role in determining amount and purity in many manufacturing processes. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Includes kit list and. Lab Acid Base Titration.
From stock.adobe.com
Acid base titration experiment and phases of color change during Lab Acid Base Titration Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titration is a common laboratory technique for determining the concentration of a solute. Includes kit list and safety instructions. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Be able to. Lab Acid Base Titration.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Lab Acid Base Titration Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titrations play an important role in determining amount and purity in many manufacturing processes. Titration is an analytical quantitative technique used to. Lab Acid Base Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is an analytical quantitative technique used to determine the concentration of a solute; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Includes kit list and safety instructions. Use this. Lab Acid Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Lab Acid Base Titration Includes kit list and safety instructions. Titration is a common laboratory technique for determining the concentration of a solute. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration. Lab Acid Base Titration.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 10 м A. Lab Acid Base Titration Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Titrations play an important role in determining amount and purity in many manufacturing processes. Titration is a common laboratory technique for determining the concentration of a solute. It is based on the neutralization reaction, where an acid and a base react to. Lab Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is a common laboratory technique for determining the concentration of a solute. Titrations play an important role in determining amount and purity in many manufacturing processes. Be able to determine the k a or k b from ph data associated with the titration. Lab Acid Base Titration.
From mavink.com
Acid Base Titration Lab Procedure Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is a common laboratory technique for determining the concentration of a solute. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is an analytical quantitative technique used to determine the concentration. Lab Acid Base Titration.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Lab Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is a common laboratory technique for determining the concentration of a solute. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Includes kit list and safety instructions. Use this class. Lab Acid Base Titration.
From www.studeersnel.nl
Acid Base Lab lactic acid titration lab report Acid Base Titrations Lab Acid Base Titration Titrations play an important role in determining amount and purity in many manufacturing processes. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The concentration of a solution can be determined. Lab Acid Base Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Lab Acid Base Titration Titration is a common laboratory technique for determining the concentration of a solute. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Use this class practical to. Lab Acid Base Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Lab Acid Base Titration Titration is an analytical quantitative technique used to determine the concentration of a solute; The concentration of a solution can be determined by knowing the acid and base dissociation constant. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Titration is a common laboratory technique. Lab Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab Acid Base Titration Includes kit list and safety instructions. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titrations play an important role in determining amount and purity in many. Lab Acid Base Titration.
From www.studypool.com
SOLUTION Acid Base Titration Datasheet Studypool Lab Acid Base Titration Includes kit list and safety instructions. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is a common laboratory technique for determining the concentration of a solute. Titration is an analytical quantitative technique used to determine the concentration of a solute; It is based on the. Lab Acid Base Titration.
From mungfali.com
Acid Base Titration Lab Lab Acid Base Titration Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Titration is an analytical quantitative technique used to determine the concentration of a solute; Titration is a common laboratory technique for determining the concentration of a solute. Be able to determine the k a or k. Lab Acid Base Titration.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Lab Acid Base Titration Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titrations play an important role in determining amount and purity in many manufacturing processes. Be able to determine the k a or. Lab Acid Base Titration.