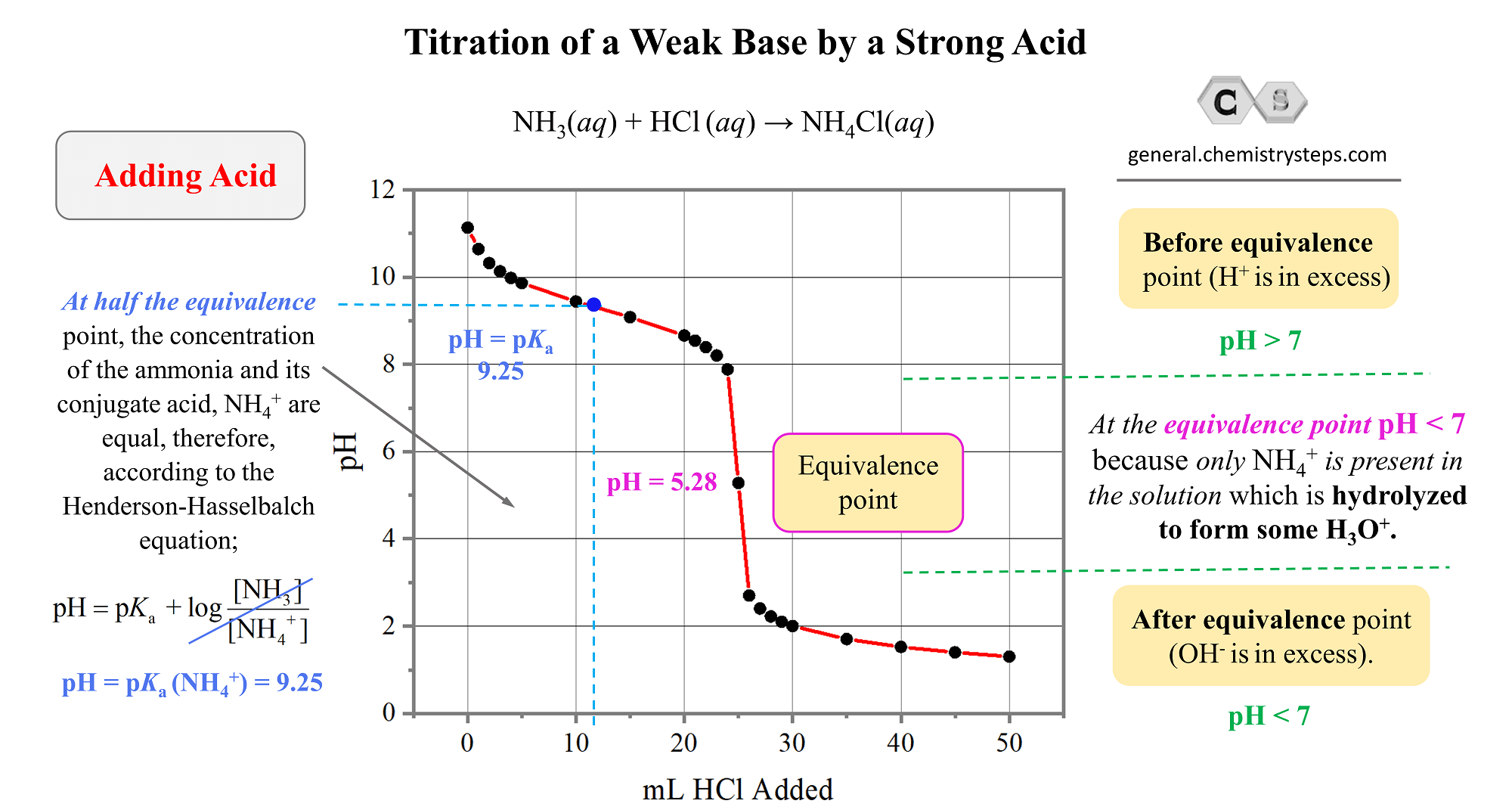
Titration Weak Base Strong Acid . in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Understand the relationship between the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. for the titration of a weak base with a strong acid, the ph curve is initially. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). The reaction of the weak acid, acetic.
from
The reaction of the weak acid, acetic. Understand the relationship between the. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. for the titration of a weak base with a strong acid, the ph curve is initially. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.
Titration Weak Base Strong Acid in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. for the titration of a weak base with a strong acid, the ph curve is initially. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. The reaction of the weak acid, acetic. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Understand the relationship between the. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7).
From
Titration Weak Base Strong Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. at what point in the titration of a weak. Titration Weak Base Strong Acid.
From schoolbag.info
Figure 10.11. Strong Acid and Weak Base Titration Curve A strong acid Titration Weak Base Strong Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a weak base with a strong acid, the ph curve is initially. Understand the relationship between the. in a titration of a weak acid/ base with a strong base/acid,. Titration Weak Base Strong Acid.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration Weak Base Strong Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. for the. Titration Weak Base Strong Acid.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Weak Base Strong Acid in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. for the titration of a weak base with a strong acid, the ph curve is initially. the titration of a weak acid with a strong base involves the direct transfer of protons from. Titration Weak Base Strong Acid.
From mungfali.com
Acid Base Titration Calculation Titration Weak Base Strong Acid at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. The reaction of the weak acid, acetic. for the titration of a weak base with a strong acid, the ph curve is initially. the titration of a weak acid with a strong base. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid Understand the relationship between the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. The reaction of the weak. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Understand the relationship between the. for the. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially,. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. The reaction of the weak acid, acetic. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. Understand the relationship between the.. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. The reaction of the weak acid, acetic. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid The reaction of the weak acid, acetic. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. . Titration Weak Base Strong Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Weak Base Strong Acid Understand the relationship between the. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. The reaction of. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid Understand the relationship between the. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. for the titration of a weak base with a strong acid, the ph curve is initially basic. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. Understand the relationship between the. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). The reaction of the weak acid, acetic. the titration of. Titration Weak Base Strong Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). the titration of a weak acid with a strong base involves the direct transfer of. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. for the titration of a weak base with a strong acid, the ph curve is initially. in a titration of a weak acid/ base with a strong. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. Understand the relationship between the. The reaction of the weak acid, acetic. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and.. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic. at what. Titration Weak Base Strong Acid.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID2134619 Titration Weak Base Strong Acid Understand the relationship between the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. The reaction of the weak. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. Understand the relationship between the. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Understand the relationship between the. for the. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a weak base with a strong acid, the ph curve is initially. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is. Titration Weak Base Strong Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially,. Titration Weak Base Strong Acid.
From mavink.com
Conductometric Titration Graph Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). Understand the relationship between the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. at what. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a flat part of the. Understand the relationship between the. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. The reaction of the. Titration Weak Base Strong Acid.
From studylib.net
Titration Curve weak base with strong acid START Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially. Understand the relationship between the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a weak base with a strong acid, the. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid Understand the relationship between the. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic. for the titration of a weak base with a strong acid, the ph curve is initially. at what point in the. Titration Weak Base Strong Acid.
From saylordotorg.github.io
AcidBase Titrations Titration Weak Base Strong Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). Understand the relationship between the. at what. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic equivalence point (ph < 7). The reaction of the weak acid, acetic. Understand the relationship between the. in a titration of a weak acid/ base with a strong base/acid, the ph changes slowly initially, then reaches a. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid The reaction of the weak acid, acetic. Understand the relationship between the. at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. for the titration of a weak base with a strong acid, the ph curve is initially. Titration Weak Base Strong Acid.
From
Titration Weak Base Strong Acid at what point in the titration of a weak acid (analyte) with a strong base (titrant) is the ph calculated using the concentration of the weak acid’s conjugate base and. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Understand the relationship between. Titration Weak Base Strong Acid.