Microbial Test Validation . this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. validation of microbiological methods likely to be used in future epa methods. criteria for validation and quality assurance in microbiological testing. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. the iso 16140 series is dedicated to the validation and verification of microbiological methods. This document is designed to be applicable to. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for.
from www.slideshare.net
this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. This document is designed to be applicable to. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. criteria for validation and quality assurance in microbiological testing. validation of microbiological methods likely to be used in future epa methods. the iso 16140 series is dedicated to the validation and verification of microbiological methods. this white paper covers what you should consider when implementing a microbial identification system, including ensuring data.
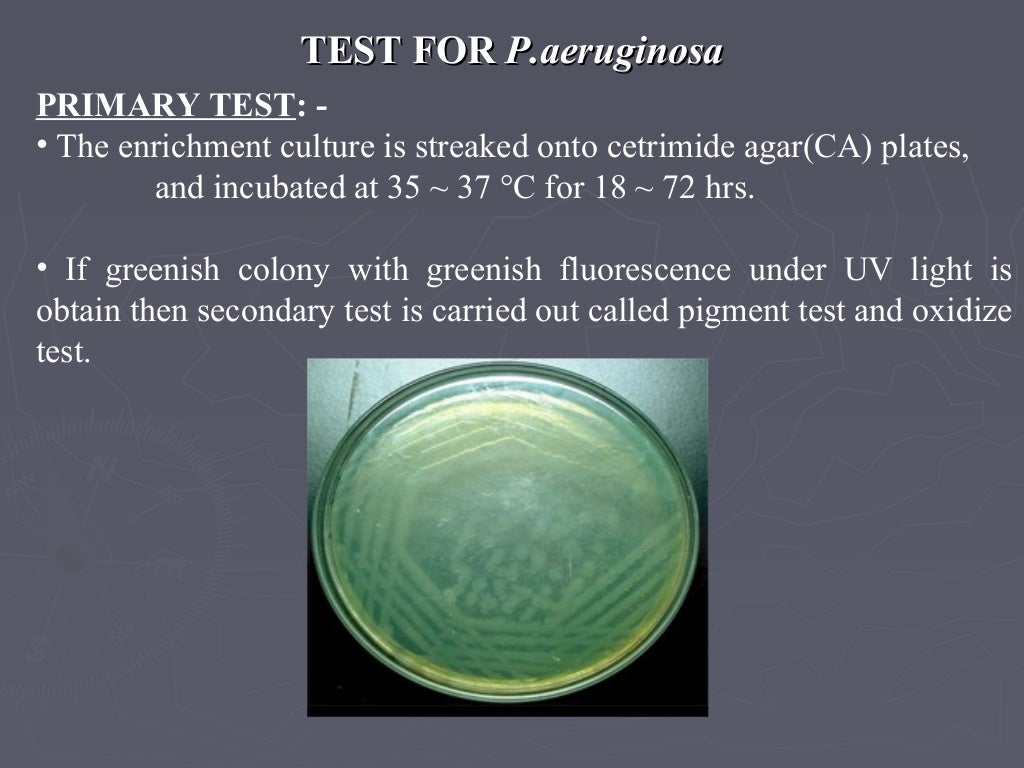
Microbial limit test
Microbial Test Validation This document is designed to be applicable to. this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. the iso 16140 series is dedicated to the validation and verification of microbiological methods. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. criteria for validation and quality assurance in microbiological testing. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. This document is designed to be applicable to. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. validation of microbiological methods likely to be used in future epa methods.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Microbial Test Validation this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this white paper covers what you should consider. Microbial Test Validation.
From www.mdpi.com
Antibiotics Free FullText Antimicrobial Susceptibility Testing A Microbial Test Validation development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the iso 16140 series is dedicated to the validation and verification of microbiological methods. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this paper aims to provide practical guidance for validating or. Microbial Test Validation.
From pharmastate.academy
SOP for Microbial Limit Test to Estimate Viable Aerobic Micro Organisms Microbial Test Validation the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. criteria for validation and quality assurance in microbiological testing. the iso 16140 series is dedicated to the validation and verification of microbiological methods. This document is designed to be applicable to. this white paper covers what you should consider. Microbial Test Validation.
From www.honeymanlaboratories.com
Water Testing Labs for Pharmaceutical Manufacture Microbial Test Validation development of standardized validation requirements for all regulatory methods used in our laboratories to detect. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. This document is designed to be applicable to. . Microbial Test Validation.
From www.slideserve.com
PPT Practical aspects of Microbiological Testing and handling of OOS Microbial Test Validation the iso 16140 series is dedicated to the validation and verification of microbiological methods. this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. This document is designed to be applicable to. criteria. Microbial Test Validation.
From ceqkqprc.blob.core.windows.net
Microbial Test Used To Identify Disease at Thaddeus Crane blog Microbial Test Validation development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. validation of microbiological methods likely to be used in future epa methods. the iso 16140 series is dedicated to the validation and verification of. Microbial Test Validation.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Test Validation the iso 16140 series is dedicated to the validation and verification of microbiological methods. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. this white paper covers what you should. Microbial Test Validation.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Test Validation this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the variety of microbiological tests makes it difficult,. Microbial Test Validation.
From www.youtube.com
section 2 Microbial Limit Test YouTube Microbial Test Validation this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. This document is designed to. Microbial Test Validation.
From www.slideshare.net
Microbial limit test Microbial Test Validation criteria for validation and quality assurance in microbiological testing. the iso 16140 series is dedicated to the validation and verification of microbiological methods. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. This document is designed to be applicable to. validation of microbiological methods likely to be used in. Microbial Test Validation.
From www.studocu.com
Microbial limit test MLT (Microbial Limit Test) Validation Learn how Microbial Test Validation criteria for validation and quality assurance in microbiological testing. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. this chapter provides guidelines for the validation of methods for the estimation of the. Microbial Test Validation.
From ceblmjfs.blob.core.windows.net
Laboratory Incubation Method at Nancy Hayes blog Microbial Test Validation this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. criteria for validation and quality assurance in microbiological testing. validation of microbiological methods likely to be used in future epa methods. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. development. Microbial Test Validation.
From www.chemwifi.com
Analytical Method validation for Pesticides, PCBs, PAHs Phthalates Microbial Test Validation the iso 16140 series is dedicated to the validation and verification of microbiological methods. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. This document is designed to be applicable to. this chapter provides. Microbial Test Validation.
From www.slideshare.net
Microbial limit test Microbial Test Validation This document is designed to be applicable to. the iso 16140 series is dedicated to the validation and verification of microbiological methods. criteria for validation and quality assurance in microbiological testing. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. development of standardized validation requirements for. Microbial Test Validation.
From www.researchgate.net
(PDF) VALIDATION OF MICROBIOLOGICAL TESTING METHODS Microbial Test Validation the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. validation of microbiological methods likely to be used in future epa methods. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. development of standardized validation requirements for all regulatory. Microbial Test Validation.
From www.americanpharmaceuticalreview.com
Live, Stressed, and Dead Their Role in Microbial Test Microbial Test Validation This document is designed to be applicable to. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. validation of microbiological methods likely to be used in future epa methods. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this paper aims to. Microbial Test Validation.
From www.slideshare.net
Microbial limit test Microbial Test Validation development of standardized validation requirements for all regulatory methods used in our laboratories to detect. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. the iso 16140 series is dedicated to the validation and verification of microbiological methods. validation of microbiological methods likely to be used. Microbial Test Validation.
From mavink.com
Product Shelf Life Chart Microbial Test Validation this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. criteria for validation and quality assurance in microbiological testing. This document is designed to be applicable to. validation of microbiological methods likely. Microbial Test Validation.
From www.researchgate.net
(PDF) VALIDATION OF MICROBIAL RECOVERY OF PHARMA CEUTICALLY IMPORTANT Microbial Test Validation this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. This document is designed to be applicable to. validation of microbiological methods likely to be used in future epa methods. the iso 16140 series is dedicated to the validation and verification of microbiological methods. development of standardized validation requirements. Microbial Test Validation.
From www.slideshare.net
Microbial limit tests I.P By Dr.P.Srinivas Jangaon institute of pharm… Microbial Test Validation the iso 16140 series is dedicated to the validation and verification of microbiological methods. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. This document is designed to be applicable to. validation. Microbial Test Validation.
From www.americanpharmaceuticalreview.com
Live, Stressed, and Dead Their Role in Microbial Test Microbial Test Validation This document is designed to be applicable to. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the iso 16140 series is dedicated to the validation and verification of microbiological methods. criteria for validation and quality assurance in microbiological testing. this paper aims to provide practical guidance for validating or. Microbial Test Validation.
From www.mdpi.com
Diagnostics Free FullText Are SemiQuantitative Clinical Cultures Microbial Test Validation this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. This document is designed to be applicable to. criteria for validation and quality assurance in microbiological testing. validation of microbiological. Microbial Test Validation.
From pharmablog.in
Microbial Limit Test for Raw Materials SOP PharmaBlog Microbial Test Validation development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. This document is designed to be applicable to. criteria for validation and quality assurance in microbiological testing. this chapter provides guidelines for the validation. Microbial Test Validation.
From www.slideshare.net
Simple way to understand Microbial Limit Test Microbial Test Validation the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. criteria for validation and quality assurance in microbiological testing. validation of microbiological methods likely to be used in future epa methods. this. Microbial Test Validation.
From www.scribd.com
Cleaning ValidationSwab Test Sample PDF Accuracy And Precision Microbial Test Validation the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. This document is designed to be applicable to. validation of microbiological methods likely to be used in future epa methods. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. this paper aims to. Microbial Test Validation.
From www.researchgate.net
Analytical and clinical validation of a microbial cellfree DNA Microbial Test Validation this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this white paper covers what. Microbial Test Validation.
From www.studocu.com
Microbial limit test validation protocol compress askaboutvalidation Microbial Test Validation the iso 16140 series is dedicated to the validation and verification of microbiological methods. this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. validation of microbiological methods likely to. Microbial Test Validation.
From www.semanticscholar.org
Table 1 from AOAC International methods committee guidelines for Microbial Test Validation this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. This document is designed to be applicable to. this chapter provides guidelines for the validation of methods for the estimation of the number of. Microbial Test Validation.
From www.scribd.com
Microbial Limit Test Validation Protocol PDF Growth Medium Microbial Test Validation development of standardized validation requirements for all regulatory methods used in our laboratories to detect. This document is designed to be applicable to. this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. criteria for validation and quality assurance in microbiological testing. this chapter provides guidelines for the validation. Microbial Test Validation.
From www.vrogue.co
Validation Of Microbiological Test Methods Processes vrogue.co Microbial Test Validation this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. the iso 16140 series is dedicated to the validation and verification of microbiological methods. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. validation of microbiological methods likely to be used in. Microbial Test Validation.
From www.slideserve.com
PPT Validation of Qualitative Microbiological Test Methods PowerPoint Microbial Test Validation This document is designed to be applicable to. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. validation of microbiological methods likely to be used in future epa methods. . Microbial Test Validation.
From pharmablog.in
Microbial Limit Test For Primary Packaging Materials SOP PharmaBlog Microbial Test Validation development of standardized validation requirements for all regulatory methods used in our laboratories to detect. the iso 16140 series is dedicated to the validation and verification of microbiological methods. This document is designed to be applicable to. validation of microbiological methods likely to be used in future epa methods. this white paper covers what you should. Microbial Test Validation.
From njlabs.com
Microbiological Testing Lab NJ Labs Microbial Test Validation criteria for validation and quality assurance in microbiological testing. the iso 16140 series is dedicated to the validation and verification of microbiological methods. development of standardized validation requirements for all regulatory methods used in our laboratories to detect. This document is designed to be applicable to. this paper aims to provide practical guidance for validating or. Microbial Test Validation.
From www.slideserve.com
PPT Validation of Qualitative Microbiological Test Methods PowerPoint Microbial Test Validation validation of microbiological methods likely to be used in future epa methods. the variety of microbiological tests makes it difficult, if not impossible, to prescribe a single, comprehensive method. this white paper covers what you should consider when implementing a microbial identification system, including ensuring data. the iso 16140 series is dedicated to the validation and. Microbial Test Validation.
From microbiologyguideritesh.com
Fundamentals of Microbial Limit Test 2023 Microbial Test Validation the iso 16140 series is dedicated to the validation and verification of microbiological methods. this paper aims to provide practical guidance for validating or verifying microbiology tests in a clinical. this chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for. This document is designed to be applicable to.. Microbial Test Validation.