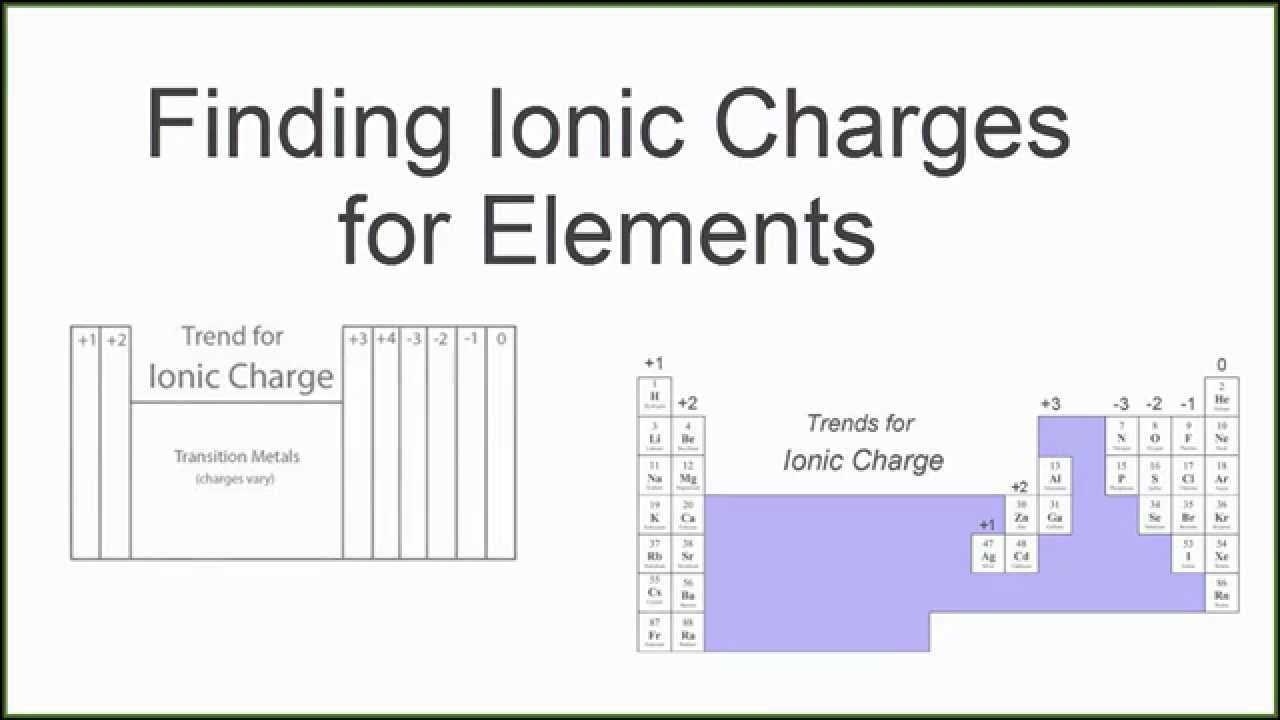
Tin Ion Charge . this table shows the most common charges for atoms of the chemical elements. Tin can have a +2 or +4 depending on how it reacts. it is defined as being the charge that an atom would have if all bonds were ionic. what is the charge of sn? a chart of cations and anions with their names, formulas and charges. You can use this table to predict whether an atom can. It slowly dissolves in dilute nonoxidizing acids or more readily. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. Metallic tin is soft and malleable. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). Uncombined elements have an oxidation.
from officialbruinsshop.com
It slowly dissolves in dilute nonoxidizing acids or more readily. Metallic tin is soft and malleable. Uncombined elements have an oxidation. this table shows the most common charges for atoms of the chemical elements. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. a chart of cations and anions with their names, formulas and charges. You can use this table to predict whether an atom can. Tin can have a +2 or +4 depending on how it reacts. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. what is the charge of sn?
Printable Periodic Table With Ionic Charges Bruin Blog
Tin Ion Charge learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). You can use this table to predict whether an atom can. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). what is the charge of sn? To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. this table shows the most common charges for atoms of the chemical elements. a chart of cations and anions with their names, formulas and charges. Uncombined elements have an oxidation. it is defined as being the charge that an atom would have if all bonds were ionic. Tin can have a +2 or +4 depending on how it reacts. Metallic tin is soft and malleable. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. It slowly dissolves in dilute nonoxidizing acids or more readily.
From officialbruinsshop.com
Printable Periodic Table With Ionic Charges Bruin Blog Tin Ion Charge Metallic tin is soft and malleable. Uncombined elements have an oxidation. You can use this table to predict whether an atom can. it is defined as being the charge that an atom would have if all bonds were ionic. a chart of cations and anions with their names, formulas and charges. learn how to name and write. Tin Ion Charge.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID548628 Tin Ion Charge To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. what is the charge of sn? this table shows the most common charges for atoms of the chemical elements. It slowly dissolves in dilute nonoxidizing acids or more readily. learn how to name and write the formula of ions with variable. Tin Ion Charge.
From correo.muycomputer.com
Determining The Ionic Charge Worksheet Printable Kids Entertainment Tin Ion Charge Uncombined elements have an oxidation. It slowly dissolves in dilute nonoxidizing acids or more readily. this table shows the most common charges for atoms of the chemical elements. it is defined as being the charge that an atom would have if all bonds were ionic. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. To form sn. Tin Ion Charge.
From www.youtube.com
Ion Charges GCSE Chemistry Revision YouTube Tin Ion Charge this table shows the most common charges for atoms of the chemical elements. It slowly dissolves in dilute nonoxidizing acids or more readily. it is defined as being the charge that an atom would have if all bonds were ionic. Metallic tin is soft and malleable. To form sn 2+, tin loses two electrons from its 5s 2. Tin Ion Charge.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Tin Ion Charge Tin can have a +2 or +4 depending on how it reacts. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Uncombined elements have an oxidation. this table shows the most common charges for atoms of the chemical elements. what is the charge of sn? it is defined as being. Tin Ion Charge.
From classfullredrafts.z13.web.core.windows.net
List Of Ions And Charges Tin Ion Charge To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Uncombined elements have an oxidation. this table shows the most common charges for atoms of the chemical elements. it is defined as being the charge that an atom would have if all bonds were ionic. Tin can have a +2 or +4. Tin Ion Charge.
From utedzz.blogspot.com
Common Ion Charges Periodic Table Periodic Table Timeline Tin Ion Charge Tin can have a +2 or +4 depending on how it reacts. Metallic tin is soft and malleable. it is defined as being the charge that an atom would have if all bonds were ionic. this table shows the most common charges for atoms of the chemical elements. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,.. Tin Ion Charge.
From pediabay.com
Periodic Table with Ionic Charges (Labeled Image) Pediabay Tin Ion Charge You can use this table to predict whether an atom can. this table shows the most common charges for atoms of the chemical elements. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. a chart of cations and anions with their names, formulas and charges. Uncombined elements have an oxidation. what is the charge of sn?. Tin Ion Charge.
From utedzz.blogspot.com
Periodic Table Ion Charges Periodic Table Timeline Tin Ion Charge it is defined as being the charge that an atom would have if all bonds were ionic. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. Tin can have a +2 or +4 depending on how it reacts. It slowly dissolves in dilute nonoxidizing acids or more readily. what is the charge of sn? a chart. Tin Ion Charge.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Tin Ion Charge Tin can have a +2 or +4 depending on how it reacts. Metallic tin is soft and malleable. it is defined as being the charge that an atom would have if all bonds were ionic. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. Uncombined elements have an oxidation. what is the charge of sn? It slowly. Tin Ion Charge.
From carsonmeowandrade.blogspot.com
Periodic Table With Ionic Charges Tin Ion Charge You can use this table to predict whether an atom can. Uncombined elements have an oxidation. what is the charge of sn? Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. Tin can have a +2 or +4 depending on how it reacts. learn how to name and write the formula of ions with variable charges, such. Tin Ion Charge.
From learningcampusvalverde.z21.web.core.windows.net
List Of Ions And Charges Tin Ion Charge Metallic tin is soft and malleable. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). It slowly dissolves in dilute nonoxidizing acids. Tin Ion Charge.
From sciencetrends.com
Periodic Table Ionic Charges, Name, And Mass Science Trends Tin Ion Charge Uncombined elements have an oxidation. Metallic tin is soft and malleable. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. Tin can have a +2 or +4 depending on how it reacts. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). You can use this table to. Tin Ion Charge.
From elchoroukhost.net
Periodic Table With Ionic Charges And Names Elcho Table Tin Ion Charge Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. Metallic tin is soft and malleable. Tin can have a +2 or +4 depending on how it reacts. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). a chart of cations and anions with their names, formulas. Tin Ion Charge.
From utedzz.blogspot.com
Periodic Table With Common Ionic Charges Periodic Table Timeline Tin Ion Charge Metallic tin is soft and malleable. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). this table shows the most common charges for atoms of the chemical elements. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. what is the charge of sn? Tin can. Tin Ion Charge.
From www.clutchprep.com
Periodic Table Charges Chemistry Video Clutch Prep Tin Ion Charge You can use this table to predict whether an atom can. Tin can have a +2 or +4 depending on how it reacts. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. what is the charge of sn? Metallic tin is soft and malleable. Tin(ii) and tin(iv) are cations with 2+ and. Tin Ion Charge.
From utedzz.blogspot.com
Periodic Table Transition Metals Charges Periodic Table Timeline Tin Ion Charge It slowly dissolves in dilute nonoxidizing acids or more readily. Uncombined elements have an oxidation. Metallic tin is soft and malleable. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. what is the charge of sn? You can use this table to predict whether an atom can. this table shows the. Tin Ion Charge.
From utedzz.blogspot.com
Periodic Table With Ionic Charges For Transition Metals Periodic Table Timeline Tin Ion Charge Uncombined elements have an oxidation. this table shows the most common charges for atoms of the chemical elements. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. It slowly dissolves in dilute nonoxidizing acids or more readily. a chart of cations and anions with their names, formulas and charges. Metallic tin. Tin Ion Charge.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Tin Have? Tin Ion Charge Tin can have a +2 or +4 depending on how it reacts. what is the charge of sn? To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. You can use this table to predict whether an atom can. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. this table shows. Tin Ion Charge.
From utedzz.blogspot.com
Printable Periodic Table Ionic Charges Periodic Table Timeline Tin Ion Charge Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). what is the charge of sn? Metallic tin is soft and malleable. It slowly dissolves in dilute nonoxidizing acids or more readily. this table shows the most. Tin Ion Charge.
From foptbest.weebly.com
Periodic table of elements with ion charges foptbest Tin Ion Charge learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). Metallic tin is soft and malleable. Uncombined elements have an oxidation. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. a chart of cations and anions with their names, formulas and charges. To form sn 2+, tin. Tin Ion Charge.
From knordslearning.com
Ionic Charges of All Elements (List + Periodic Table) Tin Ion Charge it is defined as being the charge that an atom would have if all bonds were ionic. Tin can have a +2 or +4 depending on how it reacts. what is the charge of sn? learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). Metallic tin. Tin Ion Charge.
From www.youtube.com
How To Determine The Charge of Elements and Ions Chemistry YouTube Tin Ion Charge To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. a chart of cations and anions with their names, formulas and charges. this table shows the most common charges for atoms of the chemical elements. Tin can have a +2 or +4 depending on how it reacts. Tin(ii) and tin(iv) are cations. Tin Ion Charge.
From mungfali.com
Periodic Table Of Elements Ionic Charges Tin Ion Charge Metallic tin is soft and malleable. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). It slowly dissolves in dilute nonoxidizing acids or more readily. it is defined as being the charge that an atom would have if all bonds were ionic. Tin(ii) and tin(iv) are cations. Tin Ion Charge.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry for Majors Tin Ion Charge what is the charge of sn? it is defined as being the charge that an atom would have if all bonds were ionic. a chart of cations and anions with their names, formulas and charges. Metallic tin is soft and malleable. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals.. Tin Ion Charge.
From lessonmagicthirties.z14.web.core.windows.net
How To Identify Charges Of Ions Tin Ion Charge Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. You can use this table to predict whether an atom can. a chart of cations and anions with their names, formulas and charges. Uncombined elements have an oxidation. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. It slowly dissolves in dilute. Tin Ion Charge.
From www.slideserve.com
PPT Chapter 9 “Chemical Names and Formulas” PowerPoint Presentation ID1484384 Tin Ion Charge it is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an oxidation. this table shows the most common charges for atoms of the chemical elements. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. It slowly dissolves in dilute nonoxidizing acids or more readily. To form sn. Tin Ion Charge.
From www.flinnsci.com
Ion Names, Formulas and Charges Chart Flinn Scientific Tin Ion Charge Metallic tin is soft and malleable. this table shows the most common charges for atoms of the chemical elements. a chart of cations and anions with their names, formulas and charges. Uncombined elements have an oxidation. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Tin can have a +2 or. Tin Ion Charge.
From officialbruinsshop.com
Printable Periodic Table With Ionic Charges Bruin Blog Tin Ion Charge what is the charge of sn? this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can. it is defined as being the charge that an atom would have if all bonds were ionic. To form sn 2+, tin loses two electrons from its. Tin Ion Charge.
From www.sliderbase.com
Ionic Compound Nomenclature Presentation Chemistry Tin Ion Charge It slowly dissolves in dilute nonoxidizing acids or more readily. a chart of cations and anions with their names, formulas and charges. it is defined as being the charge that an atom would have if all bonds were ionic. what is the charge of sn? Metallic tin is soft and malleable. this table shows the most. Tin Ion Charge.
From studylibdiana.z13.web.core.windows.net
Periodic Table Charge Chart Tin Ion Charge Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). It slowly dissolves in dilute nonoxidizing acids or more readily. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. a chart. Tin Ion Charge.
From sciencenotes.org
Periodic Table with Charges 118 Elements Tin Ion Charge Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. what is the charge of sn? It slowly dissolves in dilute nonoxidizing acids or more readily. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. it is defined as being the charge that an atom would have if all bonds were. Tin Ion Charge.
From mungfali.com
Periodic Table Of Elements Ionic Charges Tin Ion Charge It slowly dissolves in dilute nonoxidizing acids or more readily. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. Tin(ii) and tin(iv) are cations with 2+ and 4+ charges,. Metallic tin is soft and malleable. learn how to name and write the formula of ions with variable charges, such as tin (ii). Tin Ion Charge.
From www.slideserve.com
PPT Ionic Charges PowerPoint Presentation, free download ID5125917 Tin Ion Charge Uncombined elements have an oxidation. To form sn 2+, tin loses two electrons from its 5s 2 or 5p 2 orbitals. it is defined as being the charge that an atom would have if all bonds were ionic. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv).. Tin Ion Charge.
From classfullseigneur.z21.web.core.windows.net
How To Find Ionic Charges Tin Ion Charge this table shows the most common charges for atoms of the chemical elements. Metallic tin is soft and malleable. learn how to name and write the formula of ions with variable charges, such as tin (ii) and tin (iv). You can use this table to predict whether an atom can. It slowly dissolves in dilute nonoxidizing acids or. Tin Ion Charge.