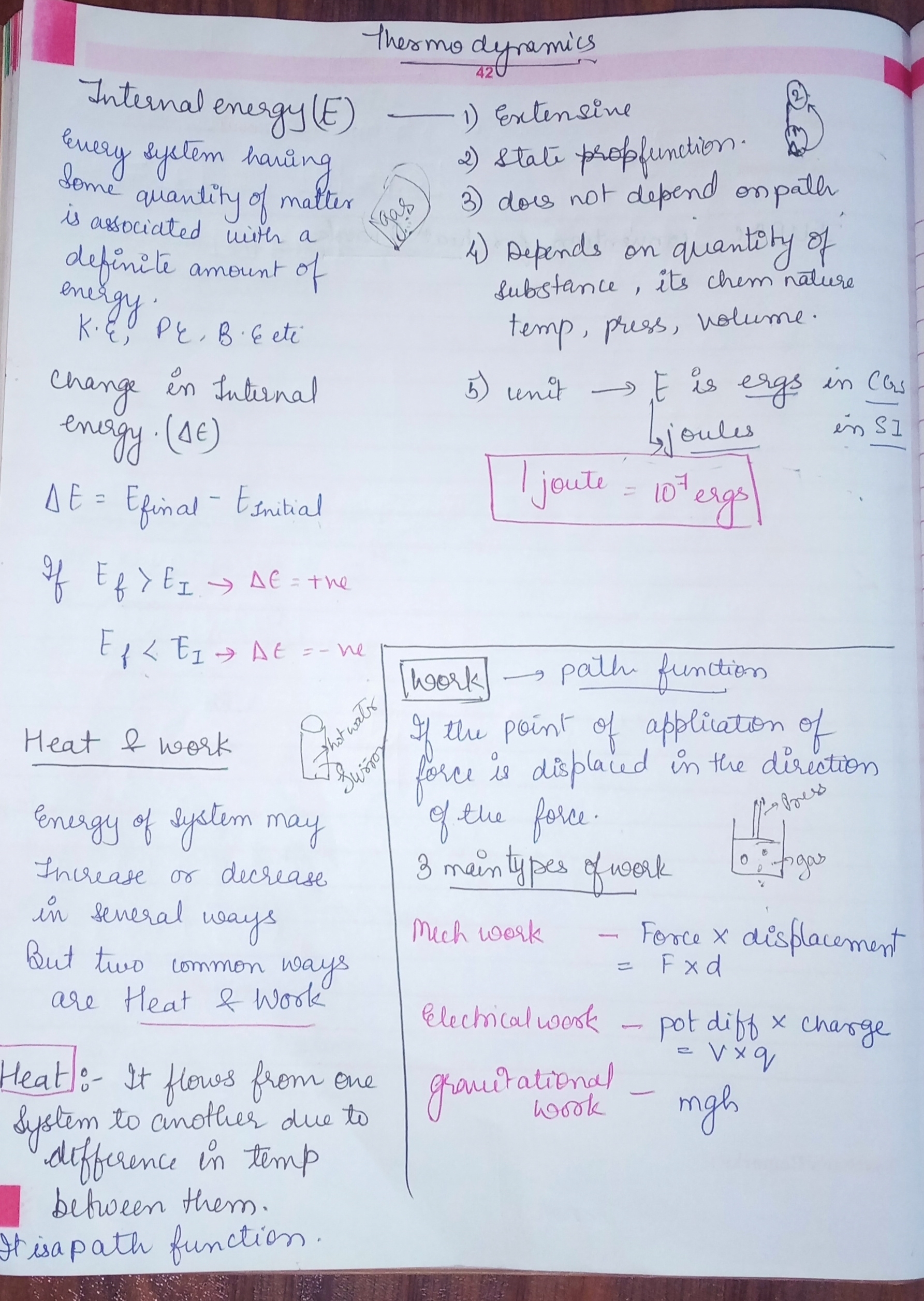
Internal Energy Measurement . Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. See how internal energy changes in. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. It is an extensive property, meaning that it depends on the amount of matter in the system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,.
from www.teachmint.com
It is an extensive property, meaning that it depends on the amount of matter in the system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. See how internal energy changes in. Internal energy is the sum of the kinetic and potential energies of the molecules in a system.
Internal Energy Chemistry Notes Teachmint
Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. See how internal energy changes in. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,.
From www.youtube.com
Internal Energy Internal Energy Physics Class 11 Internal Energy Internal Energy Measurement Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. A system’s internal energy refers to. Internal Energy Measurement.
From www.researchgate.net
Complete block diagram of energy measurement system. Download Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. Learn how internal. Internal Energy Measurement.
From www.researchgate.net
Pressure and internal energy distributions of test 4 with x0=0.4 at Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in. Internal Energy Measurement.
From www.tec-science.com
Calculation of the internal energy for ideal gases tecscience Internal Energy Measurement By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. See how internal energy changes in. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance.. Internal Energy Measurement.
From www.researchgate.net
Calculated internal energy distributions for SH fission at 274 nm Internal Energy Measurement By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. See how internal energy changes in. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u). Internal Energy Measurement.
From www.researchgate.net
Pressure and (b) specific internal energy versus the relative volume Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. See how internal energy changes in. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance.. Internal Energy Measurement.
From www.controleng.com
System1000 Flow and Energy Measurement System Control Engineering Internal Energy Measurement See how internal energy changes in. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. It is an extensive property, meaning that it depends on the amount of matter in the system. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal. Internal Energy Measurement.
From www.youtube.com
Internal Energy and First law of Thermodynamics First law of Internal Energy Measurement By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. Internal energy is the sum. Internal Energy Measurement.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of. Internal Energy Measurement.
From www.tes.com
GCSEInternal energy Teaching Resources Internal Energy Measurement By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. Learn how internal energy (u). Internal Energy Measurement.
From www.researchgate.net
Variation of geometry of internal energy with time and discharge Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. See how internal energy. Internal Energy Measurement.
From testbook.com
Internal Energy Formula Learn its Concept, Formula and Examples Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. A system’s internal energy. Internal Energy Measurement.
From pressbooks.bccampus.ca
4.1 Internal energy in a system Introduction to Engineering Internal Energy Measurement A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. See how internal energy changes in. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in. Internal Energy Measurement.
From meterhelper.com
Energy Measurement Principles Meter Helper Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. See how internal energy changes in. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal. Internal Energy Measurement.
From eduinput.com
What is Internal EnergyDefinition And Example Internal Energy Measurement A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. See how internal energy. Internal Energy Measurement.
From study.com
Internal Energy of a System Definition, Calculation & Units Lesson Internal Energy Measurement A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. It is an extensive property, meaning. Internal Energy Measurement.
From study.com
Energy Measurement Units & Conversions Video & Lesson Transcript Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. A system’s internal energy refers. Internal Energy Measurement.
From www.slideserve.com
PPT Energy Measurement Concepts PowerPoint Presentation, free Internal Energy Measurement See how internal energy changes in. It is an extensive property, meaning that it depends on the amount of matter in the system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal. Internal Energy Measurement.
From www.researchgate.net
Internal energy density e versus temperature for p1. Download Internal Energy Measurement Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. See how internal energy changes in. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Internal energy is the sum of the kinetic and potential energies of the molecules in. Internal Energy Measurement.
From www.youtube.com
Thermodynamics 4 Internal energy, Heat and Work Physical chemistry Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. A system’s internal energy refers to. Internal Energy Measurement.
From meterhelper.com
Energy Measurement Principles Meter Helper Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal energy (u) is the sum of the electronic,. Internal Energy Measurement.
From www.researchgate.net
Internal energy and heat capacity curves from MD PT (dashed line), MC Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. It is an extensive property, meaning. Internal Energy Measurement.
From www.tec-science.com
Internal energy of ideal gases tecscience Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. By measuring other properties. Internal Energy Measurement.
From www.teachmint.com
Internal Energy Chemistry Notes Teachmint Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal energy (u) is the sum of. Internal Energy Measurement.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Internal Energy Measurement A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. See how internal energy. Internal Energy Measurement.
From www.slideserve.com
PPT Energy Measurement Concepts PowerPoint Presentation, free Internal Energy Measurement Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. It is an extensive property, meaning that it depends on the amount of matter in the system. A system’s internal energy refers to. Internal Energy Measurement.
From www.youtube.com
Internal Energy GCSE Physics Revision YouTube Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in. Internal Energy Measurement.
From www.researchgate.net
Comparison of internal energy variation. Download Scientific Diagram Internal Energy Measurement A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. See how internal energy changes in. Internal energy is the sum of the kinetic and potential energies of the molecules in. Internal Energy Measurement.
From circuitglobe.com
What is Energy Meter? Definition, Construction, Working & Theory Internal Energy Measurement A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in. Internal Energy Measurement.
From krohne.company
Steam quantity measurement for internal energy balancing KROHNE Group Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. See how internal energy changes in. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in. Internal Energy Measurement.
From pressbooks.bccampus.ca
4.1 Internal energy in a system Introduction to Engineering Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. Internal energy is the sum of the kinetic and potential energies of the molecules in a system. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. Learn how internal. Internal Energy Measurement.
From www.filscihub.com
[CHEM/PHYS MODULE] GUIDE for Calculating Internal Energy (First Law of Internal Energy Measurement It is an extensive property, meaning that it depends on the amount of matter in the system. See how internal energy changes in. A system’s internal energy refers to the addition of potential and kinetic energy of that particular system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. Learn how internal energy (u). Internal Energy Measurement.
From psiberg.com
Internal Energy vs. Enthalpy Thermodynamics PSIBERG Internal Energy Measurement By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. It is an extensive property, meaning that it depends on the amount of matter in the system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. Internal energy is the sum of. Internal Energy Measurement.
From root.krohne.com
Steam quantity measurement for internal energy balancing Global Content Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. By measuring other properties like temperature, pressure, and volume, and applying the principles of thermodynamics,. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. It is an extensive property, meaning. Internal Energy Measurement.
From www.researchgate.net
Internal energy as a function of the reaction coordinate (Bryan Internal Energy Measurement Internal energy is the sum of the kinetic and potential energies of the molecules in a system. Learn how internal energy (u) is the sum of the electronic, translational, rotational, vibrational, and potential energies of molecules in a substance. It is an extensive property, meaning that it depends on the amount of matter in the system. By measuring other properties. Internal Energy Measurement.