Standard Enthalpy Of Formation Is The Change In . 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of.
from www.chegg.com
The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure.
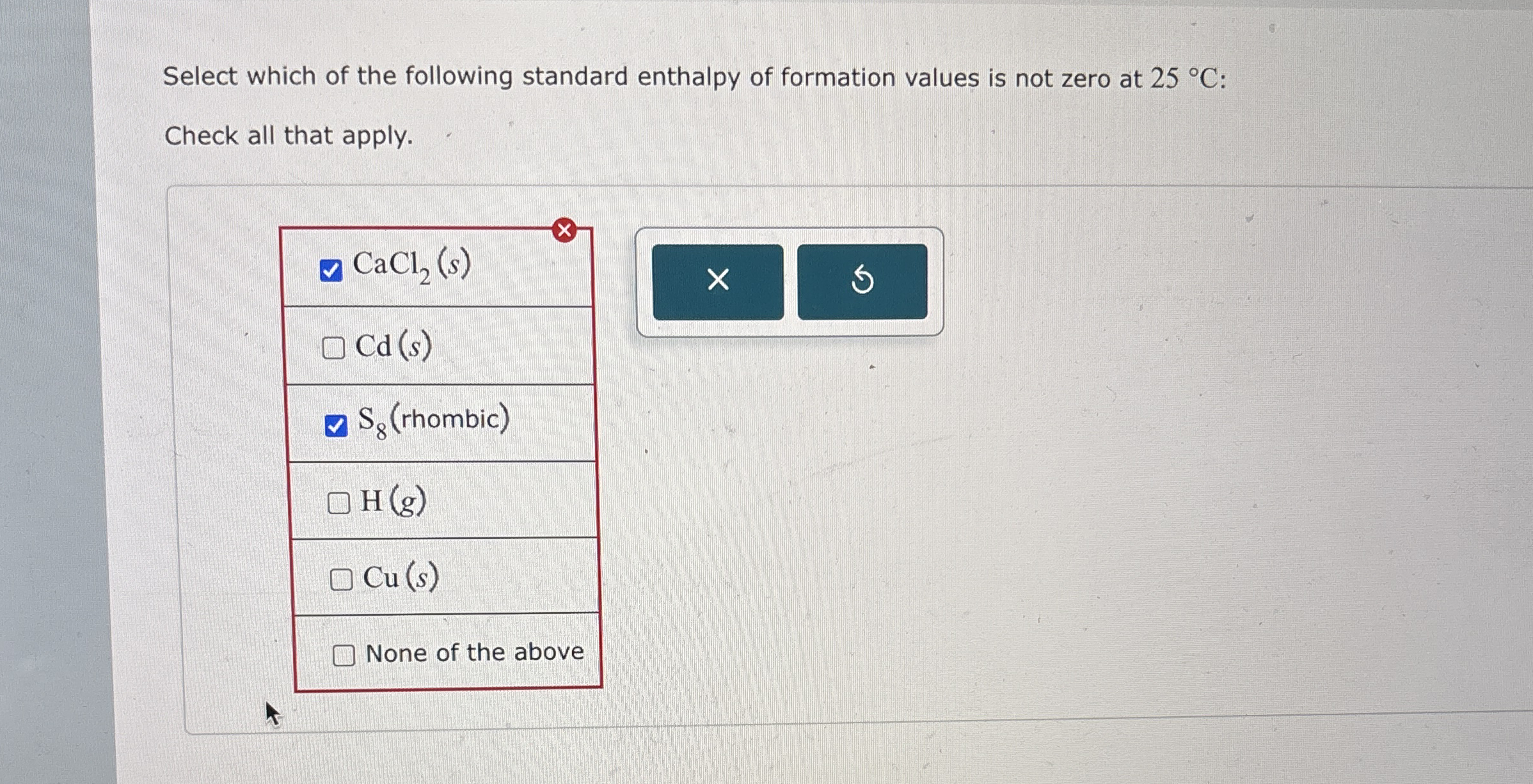
Select which of the following standard enthalpy of
Standard Enthalpy Of Formation Is The Change In The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Is The Change In Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows. Standard Enthalpy Of Formation Is The Change In.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Is The Change In A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation Is The Change In.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Is The Change In Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an. Standard Enthalpy Of Formation Is The Change In.
From ar.inspiredpencil.com
Enthalpy Of Reaction Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry. Standard Enthalpy Of Formation Is The Change In.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Is The Change In Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A standard enthalpy of formation $δh°_f$ is. Standard Enthalpy Of Formation Is The Change In.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Reaction for the Standard Enthalpy Of Formation Is The Change In Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of. Standard Enthalpy Of Formation Is The Change In.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Is The Change In A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Is The Change In.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Is The Change In Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements. Standard Enthalpy Of Formation Is The Change In.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Is The Change In The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation refer to the change in enthalpy that occurs. Standard Enthalpy Of Formation Is The Change In.
From mungfali.com
Enthalpy Change Of Formation Equation Standard Enthalpy Of Formation Is The Change In A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation. Standard Enthalpy Of Formation Is The Change In.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation Is The Change In Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation, also known as the heat of. Standard Enthalpy Of Formation Is The Change In.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Standard Enthalpy Of Formation Is The Change In Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standard enthalpy of formation (or heat of. Standard Enthalpy Of Formation Is The Change In.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of. Standard Enthalpy Of Formation Is The Change In.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Is The Change In Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry. Standard Enthalpy Of Formation Is The Change In.
From www.chegg.com
Solved The standard enthalpy change for the following Standard Enthalpy Of Formation Is The Change In Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an. Standard Enthalpy Of Formation Is The Change In.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Is The Change In The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation. Standard Enthalpy Of Formation Is The Change In.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Is The Change In The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics, the. Standard Enthalpy Of Formation Is The Change In.
From fyodbtegi.blob.core.windows.net
Standard Heat Of Formation Calcium Chloride at Lilly Owens blog Standard Enthalpy Of Formation Is The Change In A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Is The Change In.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation Is The Change In.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Enthalpy Of Formation Is The Change In Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation,. Standard Enthalpy Of Formation Is The Change In.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. The standard enthalpy of formation is. Standard Enthalpy Of Formation Is The Change In.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. A. Standard Enthalpy Of Formation Is The Change In.
From www.numerade.com
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. A standard enthalpy of. Standard Enthalpy Of Formation Is The Change In.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpies of formation. Standard Enthalpy Of Formation Is The Change In.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Is The Change In A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation \ (δh^\circ_\ce {f}\) is. Standard Enthalpy Of Formation Is The Change In.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Is The Change In Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation Is The Change In.
From www.studocu.com
Standard Enthalpy OF Formation C 2 H 2 (g) +226 H 2 SO 4 (l) −811 Standard Enthalpy Of Formation Is The Change In A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation. Standard Enthalpy Of Formation Is The Change In.
From www.hotzxgirl.com
Enthalpy Of Formation Equation Hot Sex Picture Standard Enthalpy Of Formation Is The Change In The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation \. Standard Enthalpy Of Formation Is The Change In.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Is The Change In Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of. Standard Enthalpy Of Formation Is The Change In.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.2 Standard Enthalpy Change翰林国际教育 Standard Enthalpy Of Formation Is The Change In 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. The standard enthalpy of formation is defined as the change in enthalpy when. Standard Enthalpy Of Formation Is The Change In.
From www.nagwa.com
Question Video Using Standard Enthalpies of Formation to Find Δ퐻⦵ in Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Standard enthalpies of formation refer to the change in enthalpy that occurs when one mole of a compound is formed from its elements in. A standard enthalpy of formation $δh°_f$. Standard Enthalpy Of Formation Is The Change In.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Of Formation Is The Change In The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Enthalpy of formation (δhf) is the. Standard Enthalpy Of Formation Is The Change In.
From www.chegg.com
Select which of the following standard enthalpy of Standard Enthalpy Of Formation Is The Change In Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon. Standard Enthalpy Of Formation Is The Change In.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Is The Change In Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements. Standard Enthalpy Of Formation Is The Change In.
From haipernews.com
How To Calculate Heat In A Reaction Haiper Standard Enthalpy Of Formation Is The Change In The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. A standard enthalpy of formation $δh°_f$. Standard Enthalpy Of Formation Is The Change In.