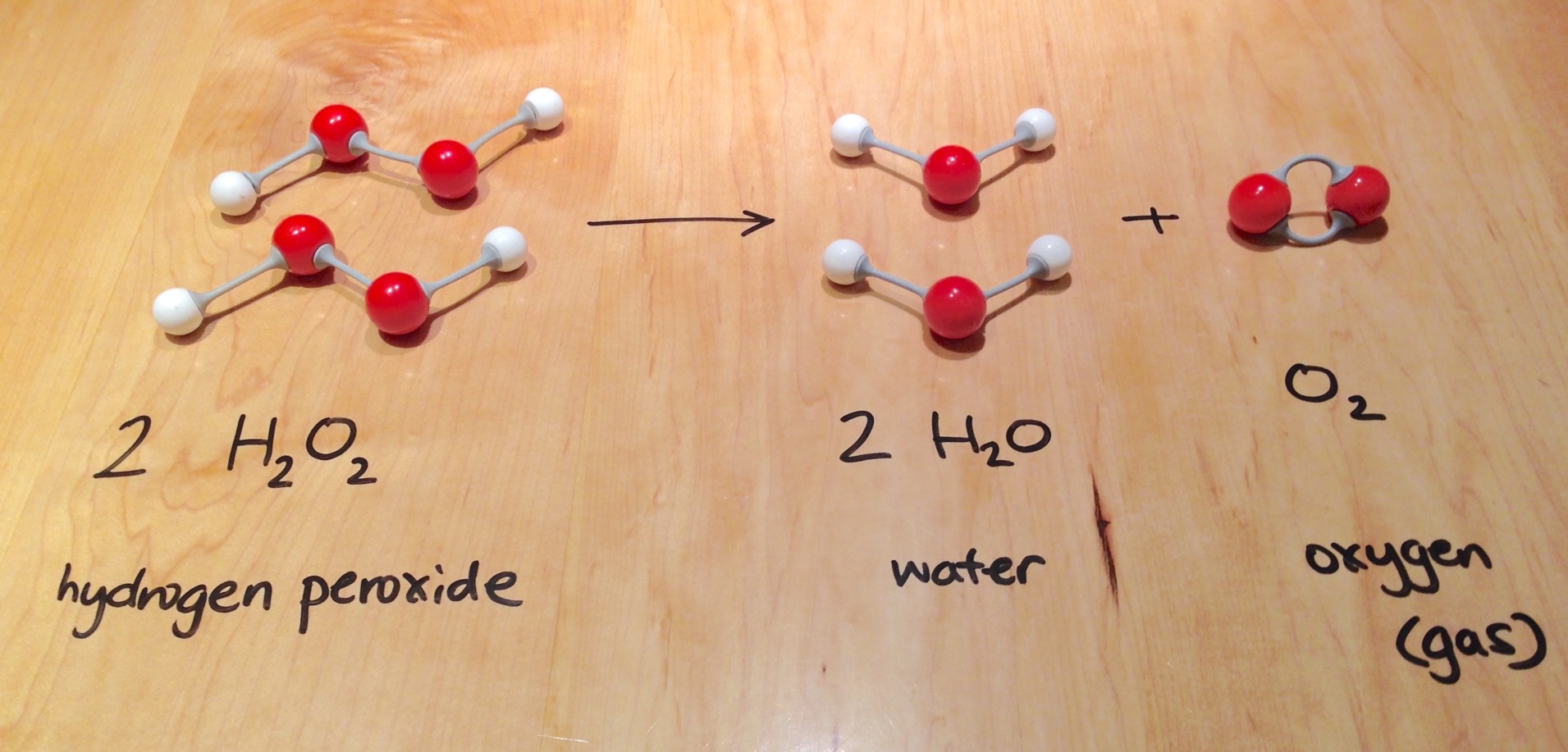
Bleach And Hydrogen Peroxide Chemical Reaction . A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). So 0.1 mol naclo is contained in 250 ml bleach. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent.
from www.ingridscience.ca
So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the.
Hydrogen peroxide chemistry ingridscience.ca
Bleach And Hydrogen Peroxide Chemical Reaction For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release.
From www.slideshare.net
Laundry Science Bleach And Hydrogen Peroxide Chemical Reaction The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. So 0.1 mol naclo is contained in 250 ml bleach. In the absence of an alkaline medium, hydrogen peroxide is. Bleach And Hydrogen Peroxide Chemical Reaction.
From issuu.com
Use of Hydrogen Peroxide as Bleaching Agent in paper and Pulp Industry Bleach And Hydrogen Peroxide Chemical Reaction For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. So 0.1 mol naclo is contained in 250 ml bleach. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the.. Bleach And Hydrogen Peroxide Chemical Reaction.
From vitngon24h.com
What Happens When You Mix Bleach And Peroxide Unveiling The Chemical Bleach And Hydrogen Peroxide Chemical Reaction Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. A 3% sodium hypochlorite contains. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.youtube.com
Textile Wet Processing1_L20 Hydrogen Peroxide Bleaching YouTube Bleach And Hydrogen Peroxide Chemical Reaction Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. So 0.1 mol naclo is contained in 250 ml bleach. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide.. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.dailymotion.com
Hydrogen Peroxide and Bleach Chemical Reaction video Dailymotion Bleach And Hydrogen Peroxide Chemical Reaction For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. So 0.1 mol naclo is contained in 250 ml bleach. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. In the. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.tessshebaylo.com
Hydrogen Peroxide And Bleach Reaction Balanced Equation Tessshebaylo Bleach And Hydrogen Peroxide Chemical Reaction So 0.1 mol naclo is contained in 250 ml bleach. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleaching with taml®. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.tessshebaylo.com
Hydrogen Peroxide And Bleach Chemical Equation Tessshebaylo Bleach And Hydrogen Peroxide Chemical Reaction Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. So 0.1 mol naclo is contained in 250 ml bleach. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. A. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.alamy.com
Hydrogen peroxide molecule. Reactive oxygen species (ROS). Used as Bleach And Hydrogen Peroxide Chemical Reaction Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. A 3%. Bleach And Hydrogen Peroxide Chemical Reaction.
From howchimp.com
Hydrogen Peroxide and Bleach What Happens When Mixed? HowChimp Bleach And Hydrogen Peroxide Chemical Reaction The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. For example, the bleaching stage of hair dyeing often employs. Bleach And Hydrogen Peroxide Chemical Reaction.
From sciencenotes.org
Oxygen Bleach vs Chlorine Bleach Bleach And Hydrogen Peroxide Chemical Reaction Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). In the absence of an alkaline medium, hydrogen. Bleach And Hydrogen Peroxide Chemical Reaction.
From beezzly.com
Hydrogen Peroxide and Bleach ⭐ All The Risks Beezzly Bleach And Hydrogen Peroxide Chemical Reaction The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. So 0.1 mol naclo is contained in 250 ml bleach.. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.tessshebaylo.com
Hydrogen Peroxide And Bleach Reaction Balanced Equation Tessshebaylo Bleach And Hydrogen Peroxide Chemical Reaction Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. So 0.1 mol naclo is contained in 250 ml bleach. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process,. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.alamy.com
Hydrogen peroxide molecule. Reactive oxygen species (ROS). Used as Bleach And Hydrogen Peroxide Chemical Reaction So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. For example, the bleaching stage of hair dyeing. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.youtube.com
Reaction of Bleaching Powder with Hydrogenperoxide, Chemistry Lecture Bleach And Hydrogen Peroxide Chemical Reaction Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). So 0.1 mol naclo is contained in 250 ml bleach. For example,. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.ingridscience.ca
Hydrogen peroxide chemistry ingridscience.ca Bleach And Hydrogen Peroxide Chemical Reaction A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. So. Bleach And Hydrogen Peroxide Chemical Reaction.
From issuu.com
How does Hydrogen Peroxide acts as a Bleaching Agent? s by Bleach And Hydrogen Peroxide Chemical Reaction Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.slideshare.net
Bleaching cotton with hydrogen peroxide Bleach And Hydrogen Peroxide Chemical Reaction Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). So 0.1. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.dreamstime.com
Hydrogen Peroxide Molecule. Reactive Oxygen Species (ROS). Used As Bleach And Hydrogen Peroxide Chemical Reaction The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. A 3% sodium hypochlorite contains. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.tessshebaylo.com
Hydrogen Peroxide And Bleach Reaction Balanced Equation Tessshebaylo Bleach And Hydrogen Peroxide Chemical Reaction A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. So 0.1 mol naclo is contained in 250 ml bleach.. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Bleach Tessshebaylo Bleach And Hydrogen Peroxide Chemical Reaction So 0.1 mol naclo is contained in 250 ml bleach. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Mixing bleach with. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Bleach Tessshebaylo Bleach And Hydrogen Peroxide Chemical Reaction Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. So 0.1 mol naclo is contained in 250 ml bleach.. Bleach And Hydrogen Peroxide Chemical Reaction.
From diymelon.com
Can You Mix Hydrogen Peroxide and Bleach? (Explained) Bleach And Hydrogen Peroxide Chemical Reaction For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. So 0.1 mol naclo is contained in 250 ml bleach. Bleaching with taml® activators and hydrogen peroxide offers another advance. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.slideshare.net
Bleaching cotton with hydrogen peroxide Bleach And Hydrogen Peroxide Chemical Reaction So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. A 3% sodium hypochlorite contains 30. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.youtube.com
Bleach and Hydrogen Peroxide Reaction + Balanced Equation YouTube Bleach And Hydrogen Peroxide Chemical Reaction A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. Bleach, sodium hypochlorite. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.alamy.com
Hydrogen peroxide molecule. Reactive oxygen species (ROS). Used as Bleach And Hydrogen Peroxide Chemical Reaction Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. So 0.1 mol naclo is contained in 250 ml bleach. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.slideserve.com
PPT Mechanical Pulping and Pulp Bleaching Chemistry PowerPoint Bleach And Hydrogen Peroxide Chemical Reaction For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process,. Bleach And Hydrogen Peroxide Chemical Reaction.
From beezzly.com
Hydrogen Peroxide and Bleach ⭐ All The Risks Beezzly Bleach And Hydrogen Peroxide Chemical Reaction For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. A 3% sodium hypochlorite contains. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.dreamstime.com
Hydrogen Peroxide Molecule. Reactive Oxygen Species (ROS). Used As Bleach And Hydrogen Peroxide Chemical Reaction Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. So 0.1 mol naclo is contained in 250 ml bleach. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. Bleaching with taml® activators and. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.youtube.com
Bleach and Hydrogen Peroxide reaction follow up YouTube Bleach And Hydrogen Peroxide Chemical Reaction The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. So 0.1 mol naclo is contained in 250 ml bleach. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). Bleaching with taml® activators and hydrogen peroxide offers another advance. Bleach And Hydrogen Peroxide Chemical Reaction.
From nctexchem.com
Bleaching Stabilizer Preparation NICCA Textile Chemicals Bleach And Hydrogen Peroxide Chemical Reaction So 0.1 mol naclo is contained in 250 ml bleach. In the absence of an alkaline medium, hydrogen peroxide is no longer effective as a bleaching agent. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. For example, the bleaching stage of hair dyeing often employs hydrogen peroxide. Bleach, sodium hypochlorite. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.youtube.com
Amazing Chemical Reaction Between Bleaching Powder & Hydrogen Peroxide Bleach And Hydrogen Peroxide Chemical Reaction The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. In the absence of. Bleach And Hydrogen Peroxide Chemical Reaction.
From giftedhouse.com
Can You Mix Bleach and Hydrogen Peroxide? Is It Safe? Bleach And Hydrogen Peroxide Chemical Reaction The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release. So 0.1 mol naclo is contained in 250 ml bleach. A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the.. Bleach And Hydrogen Peroxide Chemical Reaction.
From pubs.acs.org
Formation Mechanism of Bleaching Damage for a Biopolymer Differences Bleach And Hydrogen Peroxide Chemical Reaction Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. Bleach, sodium hypochlorite (naocl) will react with hydrogen peroxide (h2o2). A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. The nonchlorine. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.academia.edu
(PDF) The Mechanism of Hydrogen Peroxide Bleaching DURAISAMY K Bit Bleach And Hydrogen Peroxide Chemical Reaction A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. So 0.1 mol naclo is contained in 250 ml bleach. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. The nonchlorine bleaches contain hydrogen peroxide or solids like perborate or percarbonate that react with water to release.. Bleach And Hydrogen Peroxide Chemical Reaction.
From www.slideshare.net
Bleaching cotton with hydrogen peroxide Bleach And Hydrogen Peroxide Chemical Reaction A 3% sodium hypochlorite contains 30 g naclo (0.40 mol) per liter. So 0.1 mol naclo is contained in 250 ml bleach. Bleaching with taml® activators and hydrogen peroxide offers another advance in the greening of the process, by completely eliminating the. Mixing bleach with hydrogen peroxide can create a chemical reaction that produces oxygen gas and water. For example,. Bleach And Hydrogen Peroxide Chemical Reaction.