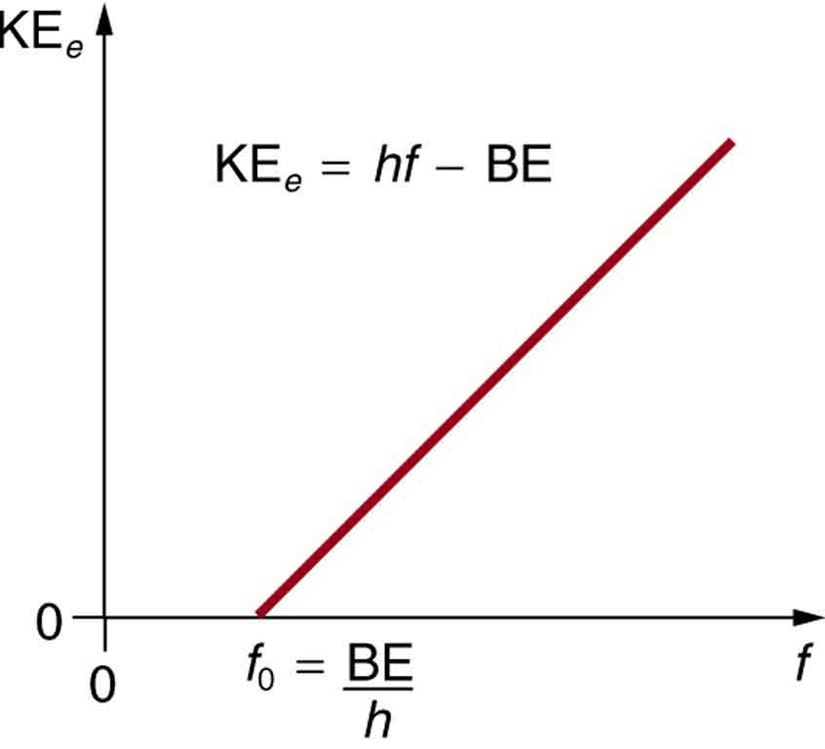
Threshold Frequency Binding Energy . (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The threshold frequency is defined as: The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold frequency. Work function (w) is the energy required to remove an electron from the material’s surface. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. In this example, we calculate the threshold frequency and binding energy from the. The work function φ, or threshold energy, of a material, is defined as: Threshold frequency (f 0) is the minimum frequency of incident light. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. Consider the electrons in a metal as. In our photoelectric effect calculator,. The minimum energy required to release a photoelectron from the surface of a metal. (b) the number of electrons.
from courses.lumenlearning.com
Work function (w) is the energy required to remove an electron from the material’s surface. Consider the electrons in a metal as. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. In our photoelectric effect calculator,. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold frequency. Threshold frequency (f 0) is the minimum frequency of incident light. The work function φ, or threshold energy, of a material, is defined as: In this example, we calculate the threshold frequency and binding energy from the. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity.
The Photoelectric Effect Physics
Threshold Frequency Binding Energy An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. The minimum energy required to release a photoelectron from the surface of a metal. Threshold frequency (f 0) is the minimum frequency of incident light. In this example, we calculate the threshold frequency and binding energy from the. (b) the number of electrons. Work function (w) is the energy required to remove an electron from the material’s surface. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The work function φ, or threshold energy, of a material, is defined as: The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. The threshold frequency is defined as: Consider the electrons in a metal as. In our photoelectric effect calculator,. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold frequency.
From www.coursehero.com
[Solved] What is the binding energy of an electron in a metal whose Threshold Frequency Binding Energy The threshold frequency is defined as: In our photoelectric effect calculator,. (b) the number of electrons. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. Threshold frequency (f 0) is the minimum frequency of incident light. Work function (w) is the energy required to remove. Threshold Frequency Binding Energy.
From www.researchgate.net
Calculation of the exciton binding energy for different dielectric Threshold Frequency Binding Energy Consider the electrons in a metal as. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. Threshold frequency (f 0) is the minimum frequency of incident light. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the. Threshold Frequency Binding Energy.
From byjus.com
If the binding energy of the electron in the ground state of hydrogen Threshold Frequency Binding Energy The minimum energy required to release a photoelectron from the surface of a metal. In this example, we calculate the threshold frequency and binding energy from the. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. Work function (w) is the energy required to remove an electron from the material’s surface. Threshold frequency (f. Threshold Frequency Binding Energy.
From www.toppr.com
CHEMISTRY e threshold frequency of the striking If the binding energy Threshold Frequency Binding Energy The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold frequency. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The work function φ, or. Threshold Frequency Binding Energy.
From www.numerade.com
SOLVEDThe binding energy of electrons in a metal is 193 kjimol. Find Threshold Frequency Binding Energy Consider the electrons in a metal as. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. In this example, we calculate the threshold frequency and binding energy from the. The. Threshold Frequency Binding Energy.
From www.shutterstock.com
Nuclear Binding Energy Curve Graph Binding Stock Illustration Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. In this example, we calculate the threshold frequency and binding energy from the. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. In our photoelectric effect calculator,. Consider. Threshold Frequency Binding Energy.
From www.researchgate.net
Binding energy spectrum of the SiO2B S sample. Download Scientific Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy). Threshold Frequency Binding Energy.
From byjus.com
Why Fe has highest binding energy and the binding energy curve Threshold Frequency Binding Energy Consider the electrons in a metal as. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the. Threshold Frequency Binding Energy.
From www.researchgate.net
Binding energy curves of the fully periodic structures of bulk graphite Threshold Frequency Binding Energy In our photoelectric effect calculator,. Work function (w) is the energy required to remove an electron from the material’s surface. Consider the electrons in a metal as. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. The threshold frequency is defined as: The quantity ϕ = h f 0 \phi = hf_0 ϕ =. Threshold Frequency Binding Energy.
From www.manminchurch.se
asistenţă Baie impuls calculate threshold frequency practică Retenţie sân Threshold Frequency Binding Energy The minimum energy required to release a photoelectron from the surface of a metal. The threshold frequency is defined as: (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. Threshold frequency (f 0) is the minimum frequency of incident light. The minimum frequency of incident. Threshold Frequency Binding Energy.
From www.researchgate.net
Molecular binding energy versus field B For each field, we Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. Work function (w) is the energy required to remove an electron from the material’s surface. The work function φ, or threshold energy, of a material, is defined as: An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy,. Threshold Frequency Binding Energy.
From www.tessshebaylo.com
Binding Energy Equation Example Tessshebaylo Threshold Frequency Binding Energy (b) the number of electrons. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. The threshold frequency is defined as: Threshold frequency (f 0) is the minimum frequency of incident light. Consider the electrons in a metal as. Work function (w) is the energy required to remove an electron from the material’s surface. (a). Threshold Frequency Binding Energy.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy Threshold Frequency Binding Energy An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. Consider the electrons in a metal as. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. In this example, we calculate. Threshold Frequency Binding Energy.
From www.researchgate.net
(a) Variation of the exciton binding energy as computed within the BSE Threshold Frequency Binding Energy An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. Threshold frequency (f 0) is the minimum frequency of incident light. Consider the electrons in a metal as. In this example, we calculate the threshold frequency and binding energy from. Threshold Frequency Binding Energy.
From www.meritnation.com
Solve this Q 19 Explain how does (i) photoelectric current and (ii Threshold Frequency Binding Energy The threshold frequency is defined as: An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. (b) the number of electrons. Threshold frequency (f 0) is the minimum frequency of incident light. Consider the electrons in a metal as. In. Threshold Frequency Binding Energy.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Frequency Binding Energy An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold. Threshold Frequency Binding Energy.
From phys.libretexts.org
10.3 Nuclear Binding Energy Physics LibreTexts Threshold Frequency Binding Energy The work function φ, or threshold energy, of a material, is defined as: Threshold frequency (f 0) is the minimum frequency of incident light. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron. Threshold Frequency Binding Energy.
From www.researchgate.net
1 The binding energy curve. 2 Crosssection of the DeuteriumTritium Threshold Frequency Binding Energy Consider the electrons in a metal as. In this example, we calculate the threshold frequency and binding energy from the. The work function φ, or threshold energy, of a material, is defined as: The minimum energy required to release a photoelectron from the surface of a metal. An individual photon of em radiation (it does not come any other way). Threshold Frequency Binding Energy.
From www.researchgate.net
Binding energy and inelastic loss in the bb channel The below Threshold Frequency Binding Energy The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed. Threshold Frequency Binding Energy.
From www.manminchurch.se
asistenţă Baie impuls calculate threshold frequency practică Retenţie sân Threshold Frequency Binding Energy The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold frequency. In our photoelectric effect calculator,. The minimum energy required to release a photoelectron from the surface of a metal. Work function (w) is the energy required to remove an. Threshold Frequency Binding Energy.
From www.chegg.com
Solved 1. The maximum energy of electrons ejected Threshold Frequency Binding Energy The minimum energy required to release a photoelectron from the surface of a metal. Consider the electrons in a metal as. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. In our photoelectric effect calculator,. Work function (w) is the energy required to remove an electron from the material’s surface. An individual photon of. Threshold Frequency Binding Energy.
From www.toppr.com
In the binding energy of electrons in a metal is 250 KJ mol 1, what Threshold Frequency Binding Energy Work function (w) is the energy required to remove an electron from the material’s surface. The work function φ, or threshold energy, of a material, is defined as: (b) the number of electrons. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. The minimum frequency. Threshold Frequency Binding Energy.
From www.slideserve.com
PPT How to Get the Binding Energy by Mass Spectrometry PowerPoint Threshold Frequency Binding Energy (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. In this example, we calculate the threshold frequency and binding energy from the. Threshold frequency (f 0) is the minimum frequency of incident light. An individual photon of em radiation (it does not come any other. Threshold Frequency Binding Energy.
From curiophysics.com
Binding Energy Per Nucleon Binding Energy Curve » Curio Physics Threshold Frequency Binding Energy (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The threshold frequency is defined as: In this example, we calculate the threshold frequency and binding energy from the. (b) the number of electrons. Threshold frequency (f 0) is the minimum frequency of incident light. Work. Threshold Frequency Binding Energy.
From www.researchgate.net
Intramolecular contribution to the polaron binding energy. 1 , 13 , 46 Threshold Frequency Binding Energy An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold frequency. In our photoelectric effect calculator,.. Threshold Frequency Binding Energy.
From www.youtube.com
Calculate the threshold frequency of metal if the binding energy is Threshold Frequency Binding Energy In this example, we calculate the threshold frequency and binding energy from the. Work function (w) is the energy required to remove an electron from the material’s surface. (b) the number of electrons. Threshold frequency (f 0) is the minimum frequency of incident light. An individual photon of em radiation (it does not come any other way) interacts with an. Threshold Frequency Binding Energy.
From www.slideserve.com
PPT 1.2 Radiation and Quantum Phenomena Quantum Threshold Frequency Binding Energy The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. (b) the number of electrons. In our photoelectric effect calculator,. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. The work function φ, or. Threshold Frequency Binding Energy.
From www.toppr.com
The threshold frequency of metal if the binding energy is 198.9 KJ mol Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. (b) the number of electrons. Work function (w) is the energy required to remove an electron from the material’s surface. The minimum frequency of incident. Threshold Frequency Binding Energy.
From www.britannica.com
Nuclear binding energy Definition, Formula, Mass Defect, & Graph Threshold Frequency Binding Energy Consider the electrons in a metal as. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. The minimum energy required to release a photoelectron from the surface of a metal. An individual photon of em radiation (it does not come any other way) interacts with. Threshold Frequency Binding Energy.
From www.tessshebaylo.com
Binding Energy Wavelength Equation Tessshebaylo Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder going. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. The threshold frequency is. Threshold Frequency Binding Energy.
From www.numerade.com
The binding energy of electrons in a metal is 193 kJ / mol . Find the Threshold Frequency Binding Energy In this example, we calculate the threshold frequency and binding energy from the. The work function φ, or threshold energy, of a material, is defined as: The minimum energy required to release a photoelectron from the surface of a metal. Consider the electrons in a metal as. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from. Threshold Frequency Binding Energy.
From courses.lumenlearning.com
The Photoelectric Effect Physics Threshold Frequency Binding Energy The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface. (a) the kinetic energy of any single emitted electron increases linearly with frequency above some threshold value and is independent of the light intensity. In this example, we calculate the threshold frequency and binding energy from the. (b) the number of electrons. The quantity ϕ. Threshold Frequency Binding Energy.
From www.researchgate.net
The H2 binding energy versus relative interatomic distance, calculated Threshold Frequency Binding Energy An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. Consider the electrons in a metal as. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break it away, with the remainder. Threshold Frequency Binding Energy.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps Threshold Frequency Binding Energy The work function φ, or threshold energy, of a material, is defined as: (b) the number of electrons. Consider the electrons in a metal as. An individual photon of em radiation (it does not come any other way) interacts with an individual electron, supplying enough energy, be, to break. The threshold frequency is defined as: The minimum energy required to. Threshold Frequency Binding Energy.
From www.meritnation.com
The binding energy of electron in a metal is 250 kJ/mol The threshold Threshold Frequency Binding Energy (b) the number of electrons. In our photoelectric effect calculator,. The work function φ, or threshold energy, of a material, is defined as: The quantity ϕ = h f 0 \phi = hf_0 ϕ = h f 0 is called the work function (expressed in units of energy) and corresponds to the threshold frequency. (a) the kinetic energy of any. Threshold Frequency Binding Energy.