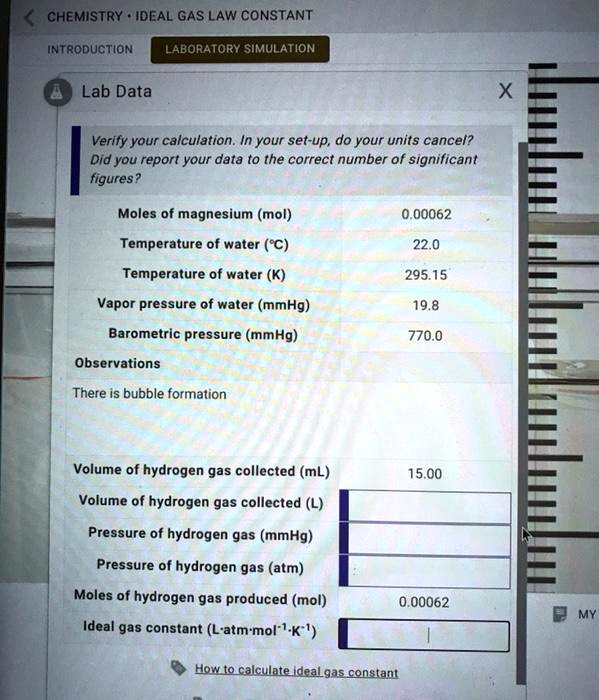
Gas Constant Lab . Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. Experimental guide to determine the value of r from the ideal gas law. Experimental guide to determine the value of r from the ideal gas law. You will measure the mass, volume, pressure, and. The ideal gas law, pv = nrt, expresses the. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas.
from www.numerade.com
Experimental guide to determine the value of r from the ideal gas law. In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. Experimental guide to determine the value of r from the ideal gas law. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. You will measure the mass, volume, pressure, and. The ideal gas law, pv = nrt, expresses the. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high.
CHEMISTRY IDEAL GAS LAW CONSTANT INTRODUCTION LABORATORY SIMULATION Lab
Gas Constant Lab In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. The ideal gas law, pv = nrt, expresses the. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. You will measure the mass, volume, pressure, and. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Experimental guide to determine the value of r from the ideal gas law. Experimental guide to determine the value of r from the ideal gas law.
From www.studocu.com
Lab 9 Evaluation of the Gas Law Constant CHEM 1411 LSC Studocu Gas Constant Lab In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental guide to determine the value of r from the ideal gas law. You will measure the mass, volume, pressure, and. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and. Gas Constant Lab.
From www.coursehero.com
[Solved] Here's the formula for ideal gas constant. Lab Data X Mass Gas Constant Lab The ideal gas law, pv = nrt, expresses the. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In this experiment, you will determine the. Gas Constant Lab.
From www.chegg.com
m ay 1060 Laboratory Experiment 6Gas Constant Fall Gas Constant Lab In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. Experimental guide to determine the value of r from the ideal gas law. In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. In this lab, students will measure various. Gas Constant Lab.
From www.studocu.com
Ex 9 Gas Constant Lab report 10 Chemistry 134L Laboratory Report Gas Constant Lab The ideal gas law, pv = nrt, expresses the. Experimental guide to determine the value of r from the ideal gas law. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. Experimental guide to determine the value of r from the ideal gas law. In this experiment. Gas Constant Lab.
From www.chegg.com
Solved Gas Constant Lab The ideal gas law, pv = nrt, expresses the. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental guide to. Gas Constant Lab.
From www.studypool.com
SOLUTION Chemistry 101 lab report universal gas constant Studypool Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental. Gas Constant Lab.
From www.chegg.com
Solved CHEMISTRY. IDEAL GAS LAW CONSTANT INTRODUCTION Gas Constant Lab In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. A gas will act like an ideal gas if its gas molecules are small,. Gas Constant Lab.
From www.coursehero.com
[Solved] Here's the formula for ideal gas constant. Lab Data X Mass Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. You will measure the mass, volume, pressure, and. Experimental guide to determine the value of r from the ideal gas law. In this lab, students. Gas Constant Lab.
From www.youtube.com
TRU Chemistry Labs First Year Experiment10 Ideal Gas Constant and Gas Constant Lab You will measure the mass, volume, pressure, and. Experimental guide to determine the value of r from the ideal gas law. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment, you will determine the value of the gas constant, r, from the. Gas Constant Lab.
From www.youtube.com
Gas Constant Lab (Concentrated HCl) YouTube Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental guide to determine the value of r from the ideal gas law. You will measure the mass, volume, pressure, and. In this. Gas Constant Lab.
From www.youtube.com
Ideal Gas Constant Lab YouTube Gas Constant Lab The ideal gas law, pv = nrt, expresses the. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental guide to determine the. Gas Constant Lab.
From www.coursehero.com
[Solved] Here's the formula for ideal gas constant. Lab Data X Mass Gas Constant Lab The ideal gas law, pv = nrt, expresses the. Experimental guide to determine the value of r from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In this experiment, you will determine the value of the gas constant, r, from the ideal. Gas Constant Lab.
From www.chegg.com
Solved Gas Constant Lab Data Sheetd) A student doing this Gas Constant Lab You will measure the mass, volume, pressure, and. Experimental guide to determine the value of r from the ideal gas law. The ideal gas law, pv = nrt, expresses the. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental guide to determine the value. Gas Constant Lab.
From www.chegg.com
Solved MISTRY IDEAL GAS LAW CONSTANT DDUCTION LABORATORY Gas Constant Lab In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Experimental guide to determine the value of r from the ideal gas law. In this lab, students will. Gas Constant Lab.
From www.chegg.com
Solved Gas Constant Lab Data Sheetd) A student doing this Gas Constant Lab In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and. Gas Constant Lab.
From www.youtube.com
Calculations for Ideal Gas Constant Lab YouTube Gas Constant Lab In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental guide to determine the value of r from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In. Gas Constant Lab.
From chem.libretexts.org
10 Experimental Determination of the Gas Constant (Experiment Gas Constant Lab In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. You will measure the mass, volume, pressure, and. A gas will act like an. Gas Constant Lab.
From www.numerade.com
CHEMISTRY IDEAL GAS LAW CONSTANT INTRODUCTION LABORATORY SIMULATION Lab Gas Constant Lab The ideal gas law, pv = nrt, expresses the. Experimental guide to determine the value of r from the ideal gas law. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases. Gas Constant Lab.
From www.chegg.com
Solved Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. Experimental guide to determine the value of r from the ideal gas law. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment, you will determine the value of the. Gas Constant Lab.
From www.chegg.com
Solved CHEMISTRY IDEAL GAS LAW CONSTANT Х À Lab Data Verify Gas Constant Lab In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Experimental guide to determine the value of r from the ideal gas. Gas Constant Lab.
From www.bioblast.at
Gas constant Bioblast Gas Constant Lab In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. Experimental guide to determine the value of r from the ideal gas law. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. Experimental. Gas Constant Lab.
From www.chegg.com
Solved CHEMISTRY. IDEAL GAS LAW CONSTANT INTRODUCTION Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. Experimental guide to determine the value of r from the ideal gas law. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. You will measure the mass, volume, pressure, and. In this. Gas Constant Lab.
From www.studypool.com
SOLUTION Determination of the Universal Gas Constant Lab Studypool Gas Constant Lab The ideal gas law, pv = nrt, expresses the. Experimental guide to determine the value of r from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. A gas will act like an ideal gas if its gas molecules are small, when the. Gas Constant Lab.
From www.youtube.com
Ideal Gas Constant Lab YouTube Gas Constant Lab The ideal gas law, pv = nrt, expresses the. Experimental guide to determine the value of r from the ideal gas law. You will measure the mass, volume, pressure, and. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Experimental guide to determine the value. Gas Constant Lab.
From www.youtube.com
Ideal Gas Equation How to Choose the Correct Gas Constant, R? With Gas Constant Lab In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. Experimental guide to determine the value of r from the ideal gas law. The ideal gas law, pv = nrt, expresses the. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure. Gas Constant Lab.
From www.chegg.com
POSTLAB Questions Help! Determining the Ideal Gas Gas Constant Lab A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. You will measure the mass, volume, pressure, and. The ideal gas law, pv =. Gas Constant Lab.
From www.scribd.com
Ideal Gas Constant Lab 2011 PDF Mole (Unit) Gases Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In. Gas Constant Lab.
From studylib.net
Experiment 6 Experimental Determination of Ideal Gas Law Constant Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. The ideal gas law, pv = nrt, expresses the. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. You will measure the mass, volume, pressure, and. A gas will act like an ideal gas. Gas Constant Lab.
From www.studocu.com
Course HERO Ideal Gas Law Constant Lab Report Determination of Ideal Gas Constant Lab In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. The ideal gas law, pv = nrt, expresses the. Experimental guide to determine the value of r from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate. Gas Constant Lab.
From www.chegg.com
Solved CHEMISTRY IDEAL GAS LAW CONSTANT SUBMIT Gas Constant Lab In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the gas. In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. Experimental guide to determine the value of r from the ideal gas law. The ideal gas law, pv. Gas Constant Lab.
From www.chegg.com
Solved Experiment 10 The Ideal Gas Law Constant (R) PostLab Gas Constant Lab Experimental guide to determine the value of r from the ideal gas law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. The ideal gas law, pv = nrt,. Gas Constant Lab.
From www.youtube.com
Gas Constant Lab (6 M Hydrochloric Acid) YouTube Gas Constant Lab A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. You will measure the mass, volume, pressure, and. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. The ideal gas law, pv =. Gas Constant Lab.
From www.youtube.com
ideal gas constant lab YouTube Gas Constant Lab Determine the value of the ideal gas constant, r, by measuring the volume, temperature, and pressure of a known amount of gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment, you will determine the value of the gas constant, r, from. Gas Constant Lab.
From www.chegg.com
REPORT SHEET Determination of R The Gas Law Constant Gas Constant Lab In this experiment, you will determine the value of the gas constant, r, from the ideal gas law. The ideal gas law, pv = nrt, expresses the. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2) calculate the gas. Determine the value of the ideal gas constant, r, by measuring. Gas Constant Lab.