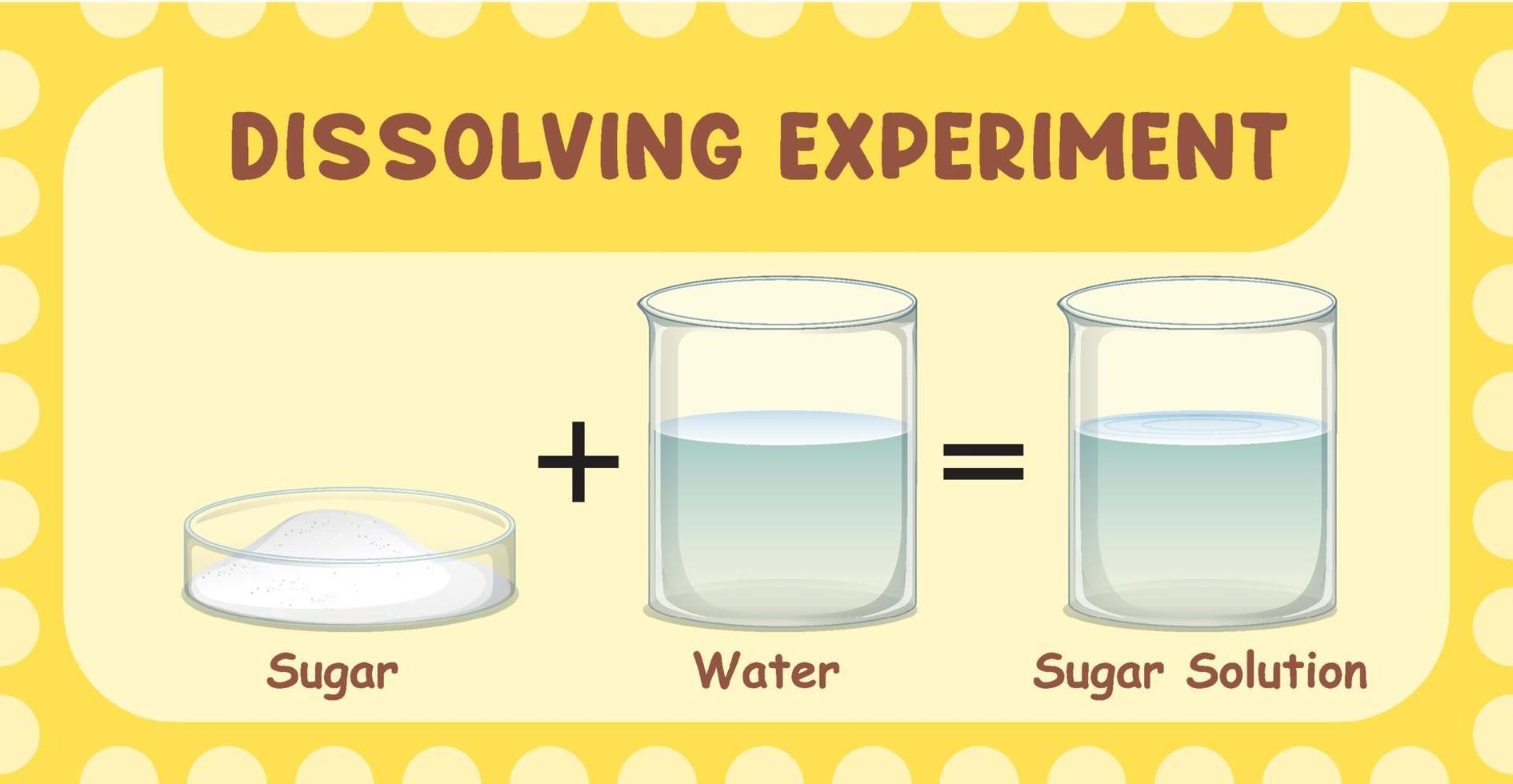
Point Where Water Can No Longer Dissolve Salt . If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. What happens when you add too much salt to water? When you add too much salt to water, the solution becomes saturated, meaning that it. The lower the temperature falls, the more. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve.
from manuallistgelsemine.z13.web.core.windows.net
Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. What happens when you add too much salt to water? The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. When you add too much salt to water, the solution becomes saturated, meaning that it. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. The lower the temperature falls, the more.
Salt Dissolving In Water Physical Or Chemical
Point Where Water Can No Longer Dissolve Salt If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water What happens when you add too much salt to water? Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. The lower the temperature falls, the more. When you add too much salt to water, the solution becomes saturated, meaning that it. The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very.
From answersfromfaq.com
Does salt dissolve better in hot or cold water? answersfromfaq/ Point Where Water Can No Longer Dissolve Salt What happens when you add too much salt to water? A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water When you add too much salt to water, the solution becomes saturated, meaning that it. Generally, a solution is considered to be saturated when it has reached the point. Point Where Water Can No Longer Dissolve Salt.
From brainly.ph
Note The 35 grams of table salt will form saturated solution in 100 ml Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more. What happens when you add too much salt to water? Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly. Point Where Water Can No Longer Dissolve Salt.
From heaventaste.org
Discovering Compatibility Does Salt Dissolve in Oil? Heaven Taste Point Where Water Can No Longer Dissolve Salt When you add too much salt to water, the solution becomes saturated, meaning that it. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. The lower the. Point Where Water Can No Longer Dissolve Salt.
From www.thoughtco.com
Dissolve Definition in Chemistry Point Where Water Can No Longer Dissolve Salt Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. Water can dissolve salt because the positive part of water molecules. Point Where Water Can No Longer Dissolve Salt.
From www.chegg.com
Solved The solubilities of three salts in water are shown in Point Where Water Can No Longer Dissolve Salt When you add too much salt to water, the solution becomes saturated, meaning that it. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. Generally, a solution is considered to be saturated when it has reached the point where no more salt. Point Where Water Can No Longer Dissolve Salt.
From stock.adobe.com
How does sodium chloride (NaCl) dissolve in water Векторный объект Point Where Water Can No Longer Dissolve Salt If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. The lower the temperature falls, the more. Water can dissolve salt. Point Where Water Can No Longer Dissolve Salt.
From www.youtube.com
What solvent can dissolve salt? YouTube Point Where Water Can No Longer Dissolve Salt Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. What happens when you add too much salt to water? A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water The lower the temperature falls, the more potassium nitrate. Point Where Water Can No Longer Dissolve Salt.
From powerupcook.com
How Does Salt Dissolve In Water Power Up Cook Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The lower the temperature falls, the more. If by salt you mean sodium. Point Where Water Can No Longer Dissolve Salt.
From curiosityguide.org
How Does Salt Dissolve in Water? Curiosity Guide Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. When you add too much salt. Point Where Water Can No Longer Dissolve Salt.
From www.greelane.com
Dissolvere il sale nell'acqua è un cambiamento chimico o fisico? Point Where Water Can No Longer Dissolve Salt If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. The lower the temperature falls, the more. The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. A machine learning model. Point Where Water Can No Longer Dissolve Salt.
From slideplayer.com
CHAPTER 2 Matter and Atoms 2.3 Mixtures and Solutions. ppt download Point Where Water Can No Longer Dissolve Salt Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to. Point Where Water Can No Longer Dissolve Salt.
From gerardonewshudson.blogspot.com
Which Best Explains Why Water Dissolves Most Salts Point Where Water Can No Longer Dissolve Salt A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. What happens when you add too much salt to water? If you keep adding more and more. Point Where Water Can No Longer Dissolve Salt.
From es.vecteezy.com
Disolver el experimento científico con azúcar se disuelve en agua Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. What happens when you add too much salt to water? A machine learning model has revealed how crystals of sodium. Point Where Water Can No Longer Dissolve Salt.
From schematiclistpact101.z22.web.core.windows.net
Diagram Of Salt Dissolved In Water Point Where Water Can No Longer Dissolve Salt What happens when you add too much salt to water? The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. The lower the temperature falls, the more. When you add too much salt to water, the solution becomes saturated, meaning that it. If by salt you mean sodium chloride, the. Point Where Water Can No Longer Dissolve Salt.
From schematiclistpact101.z22.web.core.windows.net
Salt Dissolving In Water Diagram Point Where Water Can No Longer Dissolve Salt What happens when you add too much salt to water? Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The lower the temperature falls, the more. Generally, a solution is considered to be saturated when it has reached the point where no. Point Where Water Can No Longer Dissolve Salt.
From www.chegg.com
Solved If you dissolve a substance such as ordinary table Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. When you add. Point Where Water Can No Longer Dissolve Salt.
From www.chegg.com
Solved Here are the solubilities of some salts in water.The Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. What happens when you add too much salt to water? A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water If by salt you mean sodium chloride, the dissolution. Point Where Water Can No Longer Dissolve Salt.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. The lower the temperature falls, the more. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. A machine learning model. Point Where Water Can No Longer Dissolve Salt.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Point Where Water Can No Longer Dissolve Salt Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. The lower the temperature falls, the more. The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. Water can dissolve salt because the positive part of water molecules attracts the. Point Where Water Can No Longer Dissolve Salt.
From powerupcook.com
How To Dissolve Salt Power Up Cook Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. The lower. Point Where Water Can No Longer Dissolve Salt.
From butjustwhy.com
Why does salt dissolve in water? But Just Why!? Point Where Water Can No Longer Dissolve Salt Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The lower the temperature falls, the more. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how. Point Where Water Can No Longer Dissolve Salt.
From www.youtube.com
Separate salt from water YouTube Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. If by salt you mean sodium chloride, the dissolution in that case is. Point Where Water Can No Longer Dissolve Salt.
From ar.inspiredpencil.com
Salt Dissolving In Water Point Where Water Can No Longer Dissolve Salt When you add too much salt to water, the solution becomes saturated, meaning that it. What happens when you add too much salt to water? If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. The lower the temperature falls, the more potassium nitrate crystallizes, because the water. Point Where Water Can No Longer Dissolve Salt.
From www.greelane.com
Cili është efekti i Jonit të Përbashkët? Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. When you add too much salt to water, the solution becomes saturated, meaning that it. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so. Point Where Water Can No Longer Dissolve Salt.
From ar.inspiredpencil.com
Dissolving Salt In Water Clip Art Point Where Water Can No Longer Dissolve Salt When you add too much salt to water, the solution becomes saturated, meaning that it. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions,. Point Where Water Can No Longer Dissolve Salt.
From ar.inspiredpencil.com
Dissolving In Water Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Generally, a solution is considered to be saturated when it. Point Where Water Can No Longer Dissolve Salt.
From bettertogether.org
Is Salt Dissolving In Water A Chemical Change Better Together Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. What happens when you add too much salt to water? If by salt you mean sodium chloride, the dissolution in. Point Where Water Can No Longer Dissolve Salt.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Point Where Water Can No Longer Dissolve Salt Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The lower the temperature falls, the more. If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how. Point Where Water Can No Longer Dissolve Salt.
From powerupcook.com
How To Dissolve Salt Power Up Cook Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. If you keep adding more and. Point Where Water Can No Longer Dissolve Salt.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Point Where Water Can No Longer Dissolve Salt A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. The lower the temperature falls, the more potassium nitrate crystallizes, because the. Point Where Water Can No Longer Dissolve Salt.
From powerupcook.com
Can Salt Dissolve In Oil Power Up Cook Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. If you keep adding more and more salt, eventually you will reach a point at which no more. Point Where Water Can No Longer Dissolve Salt.
From manuallistgelsemine.z13.web.core.windows.net
Salt Dissolving In Water Physical Or Chemical Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium. When you add too much salt to water, the solution becomes saturated, meaning. Point Where Water Can No Longer Dissolve Salt.
From studylib.net
Chapter 5, Lesson 3—Why Does Water Dissolve Salt? Point Where Water Can No Longer Dissolve Salt If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. When you add too much salt to water, the solution becomes saturated, meaning that it. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. The lower the. Point Where Water Can No Longer Dissolve Salt.
From www.youtube.com
What chemical will dissolve salt? YouTube Point Where Water Can No Longer Dissolve Salt If you keep adding more and more salt, eventually you will reach a point at which no more of the salt will dissolve, no matter how long or how. Generally, a solution is considered to be saturated when it has reached the point where no more salt can dissolve. A machine learning model has revealed how crystals of sodium chloride. Point Where Water Can No Longer Dissolve Salt.
From schematicfixtrysted.z22.web.core.windows.net
Diagram Of Salt Dissolving In Water Point Where Water Can No Longer Dissolve Salt The lower the temperature falls, the more potassium nitrate crystallizes, because the water will dissolve less and less of it. If by salt you mean sodium chloride, the dissolution in that case is only very slightly endothermic, so the solubility decreases very. If you keep adding more and more salt, eventually you will reach a point at which no more. Point Where Water Can No Longer Dissolve Salt.