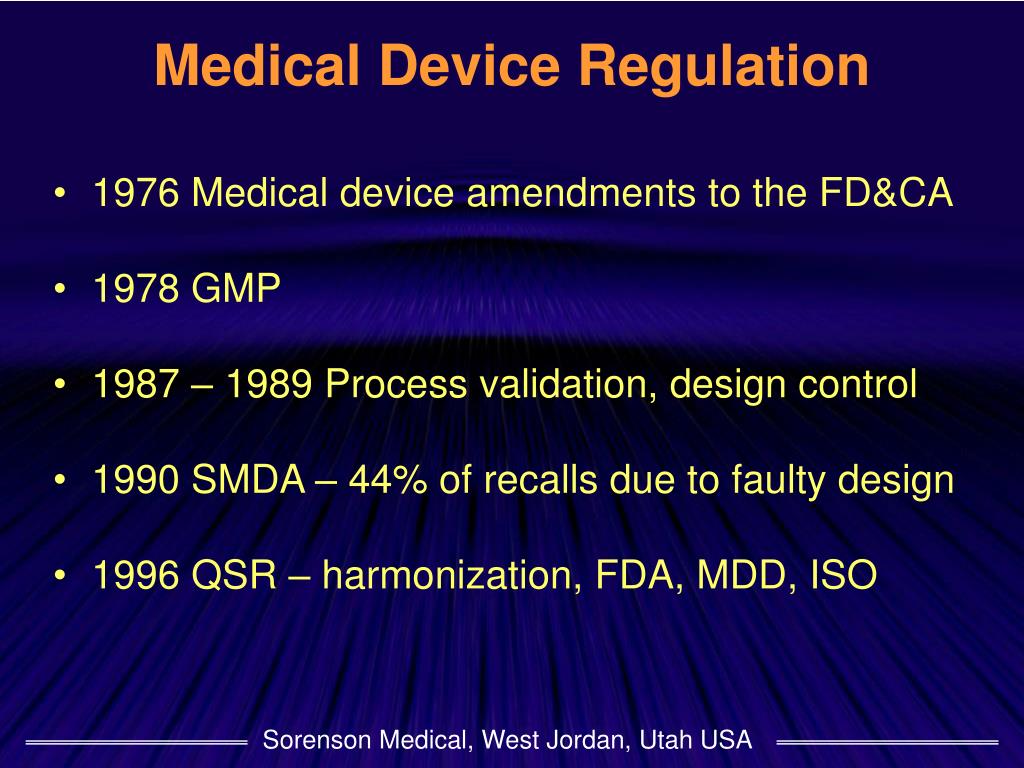
Medical Devices Defined By The Smda . in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. all new medical devices intended for the public must go through the application process overseen by the fda’s. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. act (smda) is a federal act designed to assure that all medical devices are implemented safely.
from www.slideserve.com
in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. all new medical devices intended for the public must go through the application process overseen by the fda’s. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. act (smda) is a federal act designed to assure that all medical devices are implemented safely.
PPT Medical Device Development PowerPoint Presentation, free download
Medical Devices Defined By The Smda definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. act (smda) is a federal act designed to assure that all medical devices are implemented safely. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. all new medical devices intended for the public must go through the application process overseen by the fda’s.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Defined By The Smda definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. act (smda) is a federal act designed to assure that all medical devices are implemented safely. all new medical devices intended for the public must go through the application process overseen by the fda’s. gain an understanding of the safe medical devices. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. all new medical devices intended for the public must go through the application process overseen by the fda’s. in the. Medical Devices Defined By The Smda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Devices Defined By The Smda act (smda) is a federal act designed to assure that all medical devices are implemented safely. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. all new medical devices intended for the public. Medical Devices Defined By The Smda.
From de.slideshare.net
Medical device definition Medical Devices Defined By The Smda gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. all new medical devices intended for the public must go through the application process overseen by the fda’s. the safe medical devices act (smda) of 1990 is a federal law in the united states that. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda act (smda) is a federal act designed to assure that all medical devices are implemented safely. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda all new medical devices intended for the public must go through the application process overseen by the fda’s. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. gain an. Medical Devices Defined By The Smda.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Devices Defined By The Smda in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. act (smda) is a federal act designed to assure that all medical devices are implemented safely. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. gain an understanding of the safe medical devices. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda act (smda) is a federal act designed to assure that all medical devices are implemented safely. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Device Reporting and Tracking PowerPoint Presentation Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda act (smda) is a federal act designed to assure that all medical devices are implemented safely. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda act (smda) is a federal act designed to assure that all medical devices are implemented safely. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. all new medical devices intended for the public must go through the application process overseen by the fda’s. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda act (smda) is a federal act designed to assure that all medical devices are implemented safely. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. all new medical devices intended for the public must go through the application process overseen by the fda’s. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. all new medical devices intended for the public must go through the application process overseen by the fda’s. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. gain an. Medical Devices Defined By The Smda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. act (smda) is a federal act designed to assure that all medical devices are implemented safely. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Device Development PowerPoint Presentation, free download Medical Devices Defined By The Smda definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. all new medical devices intended for the public must go through the application process overseen by the fda’s. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. in the. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. act (smda) is a federal act designed to assure that all medical devices are implemented safely. all new medical devices intended for the public must go through the application process overseen by the fda’s. the safe medical devices. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. in the 1960s and 1970s, congress responded to the public’s desire for more. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. all new medical devices intended for the public must go through the application. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. gain an. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. act (smda) is a federal act designed to assure that all medical devices are implemented safely. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. all new medical devices. Medical Devices Defined By The Smda.
From www.arenasolutions.com
Medical Device Definition Arena Medical Devices Defined By The Smda gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. all new medical devices intended for the public must go through the application. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Overview of FDA Regulation of Devices & Diagnostics PowerPoint Medical Devices Defined By The Smda in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. all new. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment PowerPoint Presentation, free download ID4432311 Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. act (smda) is a federal act designed to assure that all medical devices are implemented safely. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. the safe. Medical Devices Defined By The Smda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Defined By The Smda all new medical devices intended for the public must go through the application process overseen by the fda’s. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. in the. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. act (smda) is a federal act designed to assure that all medical devices. Medical Devices Defined By The Smda.
From dxolbfltl.blob.core.windows.net
Safe Medical Device Act List at Mildred Cruz blog Medical Devices Defined By The Smda all new medical devices intended for the public must go through the application process overseen by the fda’s. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. gain an. Medical Devices Defined By The Smda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Defined By The Smda in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. act (smda). Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. act (smda) is a federal act designed to assure that all medical devices are implemented safely. all new medical devices intended for the public must go through the application process overseen by the fda’s. gain an understanding of the safe medical devices. Medical Devices Defined By The Smda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Devices Defined By The Smda in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. act (smda) is a federal act designed to assure that all medical devices are implemented safely. . Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. act (smda) is a federal act designed to assure that all medical devices are implemented safely. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. the safe medical devices. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. in the 1960s and 1970s, congress responded to the public’s desire for more oversight over medical devices by. gain an understanding of the safe medical devices act of 1990, which established systems for the fda. Medical Devices Defined By The Smda.
From www.collidu.com
Software as a Medical Device (SaMD) PowerPoint and Google Slides Medical Devices Defined By The Smda the safe medical devices act (smda) of 1990 is a federal law in the united states that requires facilities that use medical. act (smda) is a federal act designed to assure that all medical devices are implemented safely. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor. Medical Devices Defined By The Smda.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Defined By The Smda all new medical devices intended for the public must go through the application process overseen by the fda’s. definition of a device (201(h)) an instrument, apparatus, implement, machine, contrivance implant, in vitro. gain an understanding of the safe medical devices act of 1990, which established systems for the fda to monitor the impact of. the safe. Medical Devices Defined By The Smda.