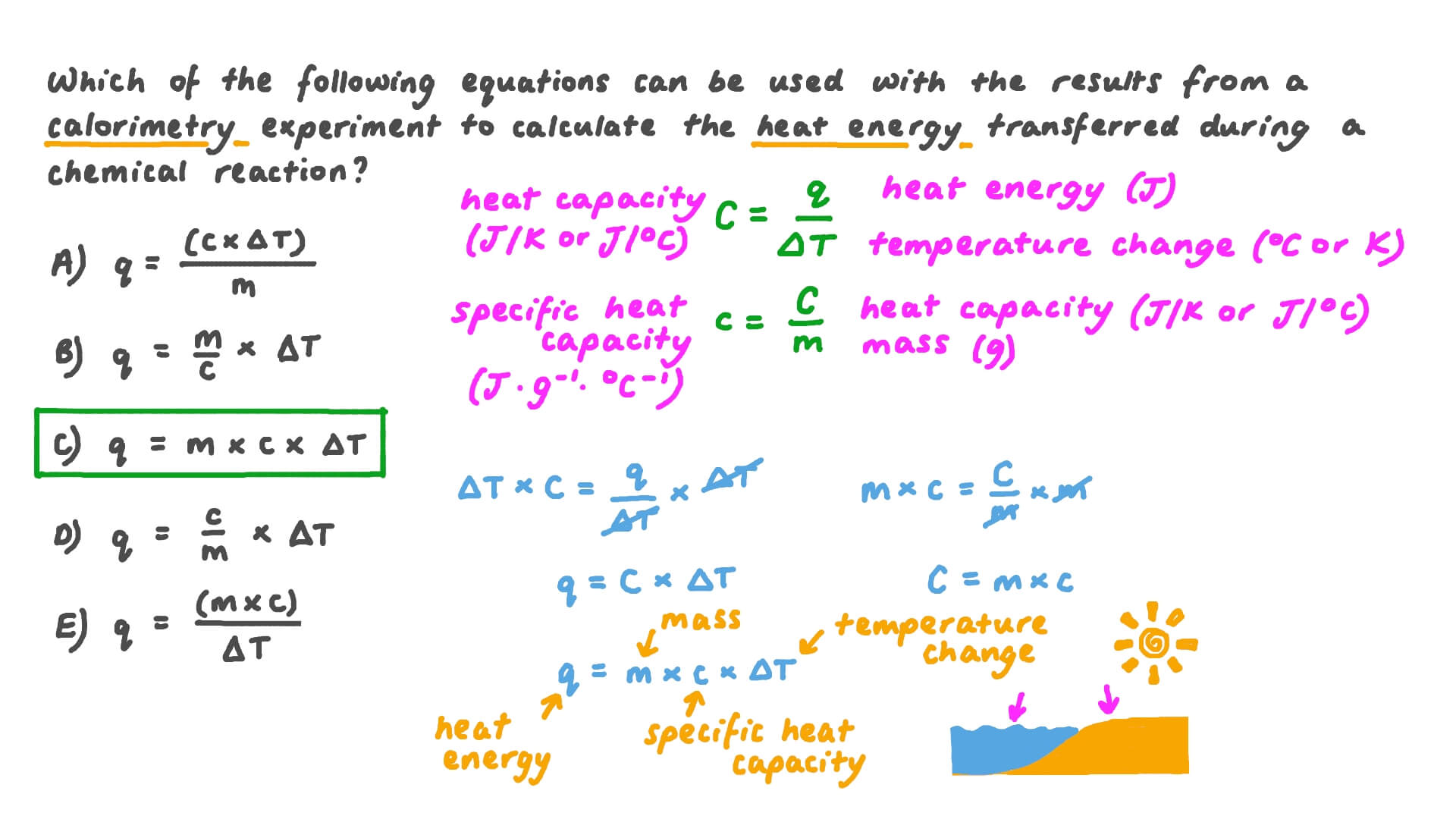How To Find The Q Of Calorimeter . State any assumptions that you made. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. Solve complex calorimetry problems with this online tool. M is the mass of the sample. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Q is the heat energy. Q = m × c × δt. Then use equation \(\pageindex{2}\) to determine the heat capacity of. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. A calorimeter constant is the. C is the specific heat capacity. Learn how to find the heat capacity of a calorimeter using different methods and examples. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Calculate the value of q for this reaction and explain the meaning of its arithmetic sign.
from fyohqoqgp.blob.core.windows.net
Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. A calorimeter constant is the. Q is the heat energy. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. The equation to calculate enthalpy changes from temperature changes is: State any assumptions that you made. Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. C is the specific heat capacity. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. M is the mass of the sample.
Calorimeter Formula In Physics at Caroline Graig blog
How To Find The Q Of Calorimeter Solve complex calorimetry problems with this online tool. The equation to calculate enthalpy changes from temperature changes is: Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. Then use equation \(\pageindex{2}\) to determine the heat capacity of. A calorimeter constant is the. C is the specific heat capacity. Learn how to find the heat capacity of a calorimeter using different methods and examples. Q is the heat energy. Q = m × c × δt. State any assumptions that you made. M is the mass of the sample. Solve complex calorimetry problems with this online tool. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How To Find The Q Of Calorimeter Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. State any assumptions that you made. M is the mass of the sample. The equation to calculate enthalpy changes from temperature changes is: Then use equation \(\pageindex{2}\) to determine the heat capacity of. C is the specific heat capacity. Learn how to determine the. How To Find The Q Of Calorimeter.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry How To Find The Q Of Calorimeter State any assumptions that you made. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. M is the mass of the sample. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Q = m × c × δt. Learn how. How To Find The Q Of Calorimeter.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent How To Find The Q Of Calorimeter A calorimeter constant is the. Q = m × c × δt. Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. The equation to calculate enthalpy changes from temperature changes is: C is the specific heat capacity. Learn how to measure the amount of heat involved in a chemical or physical process using. How To Find The Q Of Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How To Find The Q Of Calorimeter Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. Q is the heat energy. State any assumptions that you made. Learn how to find the heat capacity of a calorimeter using different methods and examples. Q = m × c × δt. Solve complex calorimetry problems with this online. How To Find The Q Of Calorimeter.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How To Find The Q Of Calorimeter The equation to calculate enthalpy changes from temperature changes is: It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. A calorimeter constant is the. Q is the heat energy. Then use equation \(\pageindex{2}\) to determine the heat capacity of. M is the mass of the sample. State any assumptions. How To Find The Q Of Calorimeter.
From www.youtube.com
09.05 Calorimetry YouTube How To Find The Q Of Calorimeter Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. M is the mass of the sample. A calorimeter constant is the. State any assumptions that you made. Learn how to find the heat capacity of a calorimeter using different methods and examples. Then use equation \(\pageindex{2}\) to determine. How To Find The Q Of Calorimeter.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry How To Find The Q Of Calorimeter C is the specific heat capacity. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Solve complex calorimetry problems with this online tool. M is the mass of the sample. Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. Q is. How To Find The Q Of Calorimeter.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog How To Find The Q Of Calorimeter Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. C is the specific heat capacity. State any assumptions that you made. It can analyze heat exchange between up to. How To Find The Q Of Calorimeter.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep How To Find The Q Of Calorimeter A calorimeter constant is the. Learn how to find the heat capacity of a calorimeter using different methods and examples. Then use equation \(\pageindex{2}\) to determine the heat capacity of. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Calculate the value of q for this reaction and explain. How To Find The Q Of Calorimeter.
From studymarxianism.z21.web.core.windows.net
How To Calculate Calorimeter How To Find The Q Of Calorimeter State any assumptions that you made. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Solve complex calorimetry problems with this online tool. C is the specific heat capacity.. How To Find The Q Of Calorimeter.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter How To Find The Q Of Calorimeter The equation to calculate enthalpy changes from temperature changes is: Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. A calorimeter constant is the. Calorimetry is an application of. How To Find The Q Of Calorimeter.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube How To Find The Q Of Calorimeter A calorimeter constant is the. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. Learn how to find the heat capacity of a calorimeter using different methods and examples. Q is the heat energy. Learn how to measure the amount of heat involved in a chemical or physical process. How To Find The Q Of Calorimeter.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube How To Find The Q Of Calorimeter Learn how to find the heat capacity of a calorimeter using different methods and examples. Solve complex calorimetry problems with this online tool. Then use equation \(\pageindex{2}\) to determine the heat capacity of. M is the mass of the sample. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee.. How To Find The Q Of Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How To Find The Q Of Calorimeter Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Learn how to find the heat capacity of a calorimeter using different methods and examples. The equation to calculate. How To Find The Q Of Calorimeter.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry How To Find The Q Of Calorimeter Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Learn how to find the heat capacity of a calorimeter using different methods and examples. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. Learn how to determine. How To Find The Q Of Calorimeter.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How To Find The Q Of Calorimeter Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. A calorimeter constant is the. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. Q = m × c × δt. Calculate the value of q rxn for benzoic acid by multiplying the. How To Find The Q Of Calorimeter.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube How To Find The Q Of Calorimeter M is the mass of the sample. Learn how to find the heat capacity of a calorimeter using different methods and examples. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. C is the specific heat capacity. Learn how to determine the heat capacity of a calorimeter by. How To Find The Q Of Calorimeter.
From www.chegg.com
Solved How to calculate the calorimeter constant How To Find The Q Of Calorimeter Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. M is the mass of the sample. The equation to calculate enthalpy changes from temperature changes is: Calculate the value of q rxn for benzoic. How To Find The Q Of Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How To Find The Q Of Calorimeter A calorimeter constant is the. Then use equation \(\pageindex{2}\) to determine the heat capacity of. Solve complex calorimetry problems with this online tool. Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of.. How To Find The Q Of Calorimeter.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter How To Find The Q Of Calorimeter M is the mass of the sample. The equation to calculate enthalpy changes from temperature changes is: Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Learn how to find the heat capacity of a calorimeter using different methods and examples. A calorimeter constant is the. Calorimetry is an application of. How To Find The Q Of Calorimeter.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube How To Find The Q Of Calorimeter M is the mass of the sample. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. Solve complex calorimetry problems with this online tool. Q = m × c. How To Find The Q Of Calorimeter.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube How To Find The Q Of Calorimeter Q = m × c × δt. A calorimeter constant is the. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Then use equation \(\pageindex{2}\) to determine the heat capacity of. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium.. How To Find The Q Of Calorimeter.
From www.youtube.com
How to find calorimeter constant YouTube How To Find The Q Of Calorimeter Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. Q = m × c × δt. Then use equation \(\pageindex{2}\) to determine the heat capacity of. State any assumptions that you made. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by. How To Find The Q Of Calorimeter.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How To Find The Q Of Calorimeter Learn how to find the heat capacity of a calorimeter using different methods and examples. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Q is the heat energy. M is the mass of the sample. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of. How To Find The Q Of Calorimeter.
From saylordotorg.github.io
Calorimetry How To Find The Q Of Calorimeter Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Learn. How To Find The Q Of Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 How To Find The Q Of Calorimeter C is the specific heat capacity. Solve complex calorimetry problems with this online tool. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. A calorimeter constant is the. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb.. How To Find The Q Of Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1875569 How To Find The Q Of Calorimeter Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. A calorimeter constant is the. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. The equation to calculate enthalpy changes from temperature changes is: Q = m × c. How To Find The Q Of Calorimeter.
From courses.lumenlearning.com
Calorimetry General Chemistry How To Find The Q Of Calorimeter A calorimeter constant is the. Solve complex calorimetry problems with this online tool. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. M is the mass of the sample. Q = m × c × δt. Calculate the value of q for this reaction and explain the meaning of. How To Find The Q Of Calorimeter.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube How To Find The Q Of Calorimeter The equation to calculate enthalpy changes from temperature changes is: It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. Learn how to find the heat capacity of a calorimeter using different methods and examples. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic. How To Find The Q Of Calorimeter.
From www.youtube.com
Principle of Calorimetry YouTube How To Find The Q Of Calorimeter A calorimeter constant is the. C is the specific heat capacity. Q = m × c × δt. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. The equation to calculate enthalpy changes from temperature changes is: Q is the heat energy. Then use equation \(\pageindex{2}\) to determine the heat capacity. How To Find The Q Of Calorimeter.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter How To Find The Q Of Calorimeter C is the specific heat capacity. M is the mass of the sample. Solve complex calorimetry problems with this online tool. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the. How To Find The Q Of Calorimeter.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube How To Find The Q Of Calorimeter Learn how to find the heat capacity of a calorimeter using different methods and examples. C is the specific heat capacity. The equation to calculate enthalpy changes from temperature changes is: Calculate the value of q for this reaction and explain the meaning of its arithmetic sign. Q is the heat energy. A calorimeter constant is the. Solve complex calorimetry. How To Find The Q Of Calorimeter.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How To Find The Q Of Calorimeter Learn how to determine the heat capacity of a calorimeter by mixing water at different temperatures and measuring the equilibrium. State any assumptions that you made. M is the mass of the sample. The equation to calculate enthalpy changes from temperature changes is: Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or. How To Find The Q Of Calorimeter.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement How To Find The Q Of Calorimeter Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. M is the mass of the sample. It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. The equation to calculate enthalpy changes from temperature changes is: Calorimetry is an application of. How To Find The Q Of Calorimeter.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube How To Find The Q Of Calorimeter It can analyze heat exchange between up to 3 objects and find enthalpy change of chemical reactions in a coffee. The equation to calculate enthalpy changes from temperature changes is: C is the specific heat capacity. Calculate the value of q rxn for benzoic acid by multiplying the mass of benzoic acid by its δh comb. Solve complex calorimetry problems. How To Find The Q Of Calorimeter.