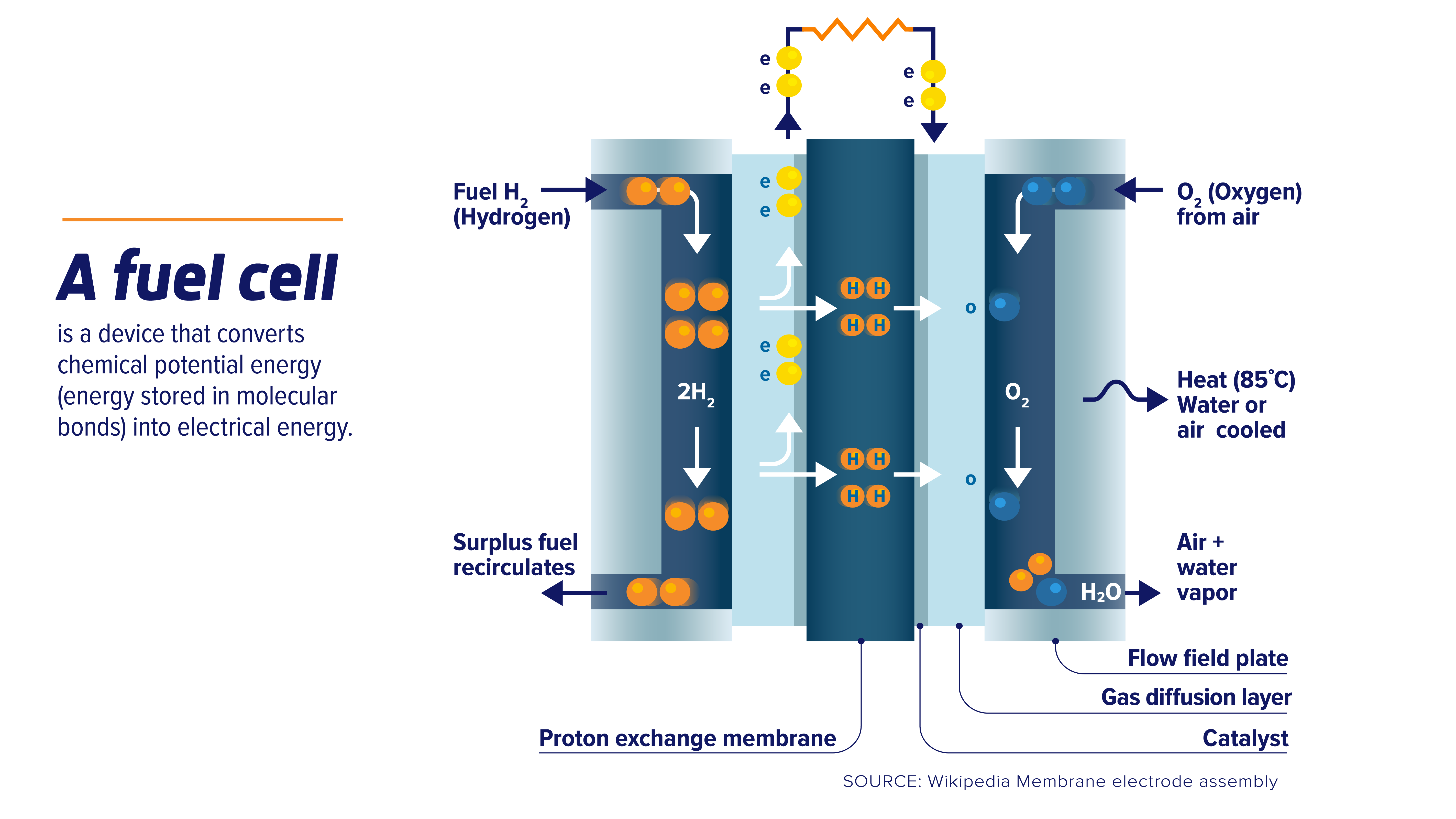
Fuel Cell Definition In Chemistry . A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. What is a fuel cell? A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing.
from www.chfca.ca
What is a fuel cell? By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical.
About Fuel Cells CHFCA
Fuel Cell Definition In Chemistry A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. What is a fuel cell? A type of galvanic cell which promises to become increasingly important in the future is the fuel cell.
From greenfuture.io
What Are Hydrogen Fuel Cell Cars? Fuel Cell Definition In Chemistry A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell can be. Fuel Cell Definition In Chemistry.
From chemistnotes.com
Fuel cell Definition, types Chemistry Notes Fuel Cell Definition In Chemistry A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell is an electrochemical device, which. Fuel Cell Definition In Chemistry.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be defined as. Fuel Cell Definition In Chemistry.
From chemistnotes.com
Fuel cell Definition, types Chemistry Notes Fuel Cell Definition In Chemistry A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell consists of two electrodes—a negative. Fuel Cell Definition In Chemistry.
From www.slideserve.com
PPT Chemistry for Fuel Cells PowerPoint Presentation, free download ID6544858 Fuel Cell Definition In Chemistry Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell is an. Fuel Cell Definition In Chemistry.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Fuel Cell Definition In Chemistry By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. What is a fuel cell? A fuel cell consists of. Fuel Cell Definition In Chemistry.
From www.myelectrical2015.com
Electrical Revolution Working Principle of Fuel Cell Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is a fuel cell? A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A type of galvanic cell which promises to become increasingly important in the future is. Fuel Cell Definition In Chemistry.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell Definition In Chemistry What is a fuel cell? A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel. Fuel Cell Definition In Chemistry.
From manualdiagramausterlitz.z19.web.core.windows.net
Fuel Cell Schematic Diagram Fuel Cell Definition In Chemistry By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell can be defined as an. Fuel Cell Definition In Chemistry.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cell Definition In Chemistry Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. By contrast to a conventional cell, where. Fuel Cell Definition In Chemistry.
From www.fuelcellstore.com
What is a Fuel Cell? Fuel Cell Definition In Chemistry A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell can be described as. Fuel Cell Definition In Chemistry.
From scienceinfo.com
Fuel Cell Definition, Types, and Advantages Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. Fuel cells are electrochemical devices. Fuel Cell Definition In Chemistry.
From library.achievingthedream.org
Phases and Classification of Matter Introductory Chemistry Lecture & Lab Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is a fuel cell? Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel. Fuel Cell Definition In Chemistry.
From siqens.de
Principle, types, advantages What is a fuel cell? SIQENS Fuel Cell Definition In Chemistry By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. What is a fuel cell? A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte.. Fuel Cell Definition In Chemistry.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is a fuel cell? A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel. Fuel Cell Definition In Chemistry.
From www.alamy.com
Fuel cell diagram. Vector. Device that converts chemical potential energy into electrical energy Fuel Cell Definition In Chemistry Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is. Fuel Cell Definition In Chemistry.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages, Disadvantages & Uses YouTube Fuel Cell Definition In Chemistry By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Fuel cells are electrochemical devices that convert chemical. Fuel Cell Definition In Chemistry.
From gamesmartz.com
Fuel Cell Definition & Image GameSmartz Fuel Cell Definition In Chemistry A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. What is a fuel cell? A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A type of galvanic cell which promises to become increasingly important in the future is. Fuel Cell Definition In Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Fuel Cell Definition In Chemistry A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel. Fuel Cell Definition In Chemistry.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Engineeringity Fuel Cell Definition In Chemistry A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is a fuel cell? A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction,. Fuel Cell Definition In Chemistry.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Fuel Cell Definition In Chemistry A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. What is a fuel cell? A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction.. Fuel Cell Definition In Chemistry.
From www.stanfordmaterials.com
What are Solid Oxide Fuel Cells? How Do They Work? Fuel Cell Definition In Chemistry A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. By contrast to a. Fuel Cell Definition In Chemistry.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. What is a fuel cell? Fuel cells. Fuel Cell Definition In Chemistry.
From www.geeksforgeeks.org
Fuel Cells Definition, Types, Advantages, Limitations Fuel Cell Definition In Chemistry What is a fuel cell? By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. Fuel cells. Fuel Cell Definition In Chemistry.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell can be described as. Fuel Cell Definition In Chemistry.
From www.thestudentroom.co.uk
Hydrogen fuel cells The Student Room Fuel Cell Definition In Chemistry A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. What is a fuel cell? By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel. Fuel Cell Definition In Chemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Fuel Cell Definition In Chemistry By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell can be defined as. Fuel Cell Definition In Chemistry.
From revisechemistry.uk
Chemical Cells and Fuel Cells AQA C5 revisechemistry.uk Fuel Cell Definition In Chemistry Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. A type of galvanic cell. Fuel Cell Definition In Chemistry.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. By contrast to. Fuel Cell Definition In Chemistry.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Fuel Cell Definition In Chemistry A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. By contrast to a conventional cell, where. Fuel Cell Definition In Chemistry.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Fuel Cell Definition In Chemistry A fuel cell can be described as an electrochemical cell which, through an electrochemical reaction, generates electrical. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. By contrast to a conventional cell, where only limited quantities of oxidizing agent and reducing. A fuel cell consists of two electrodes—a negative. Fuel Cell Definition In Chemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cell Definition In Chemistry Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. What is. Fuel Cell Definition In Chemistry.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cell Definition In Chemistry A type of galvanic cell which promises to become increasingly important in the future is the fuel cell. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell can. Fuel Cell Definition In Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell Definition In Chemistry A fuel cell is an electrochemical device, which converts the chemical energy of a fuel into electrical energy and heat. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A type. Fuel Cell Definition In Chemistry.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) Fuel Cell Definition In Chemistry A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells are electrochemical devices that convert chemical energy from fuels directly into electrical energy through an electrochemical. A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. By contrast. Fuel Cell Definition In Chemistry.