Is Copper Hydroxide Aqueous . Sat, 19 oct 2024 12:27:08 gmt simple The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The typical color of copper hydroxide is blue. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The nature of the resulting.
from www.chegg.com
The typical color of copper hydroxide is blue. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The nature of the resulting. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Sat, 19 oct 2024 12:27:08 gmt simple Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources.
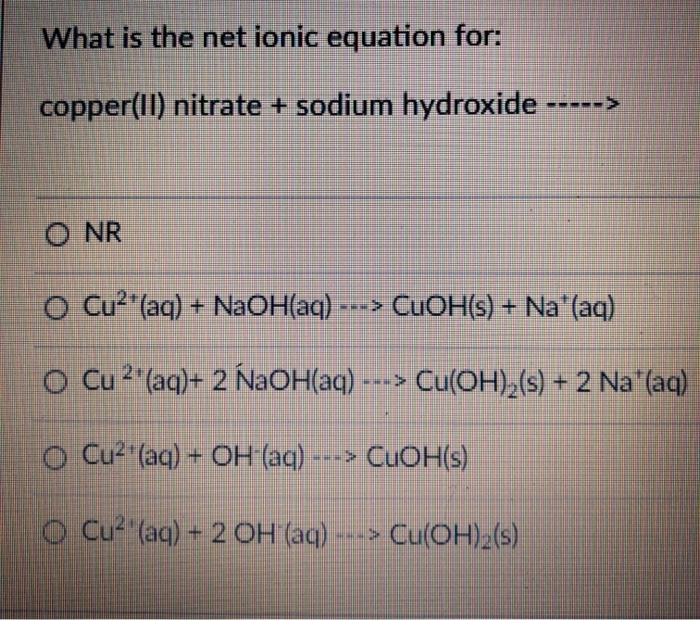
Solved What is the net ionic equation for copper(II)
Is Copper Hydroxide Aqueous The typical color of copper hydroxide is blue. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The typical color of copper hydroxide is blue. Sat, 19 oct 2024 12:27:08 gmt simple Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The nature of the resulting. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali.
From www.reddit.com
Precipitate Colors of Metal Ions in Aqueous Ammonia and Sodium Is Copper Hydroxide Aqueous Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The nature of the resulting. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Sat, 19. Is Copper Hydroxide Aqueous.
From woelen.homescience.net
Science made alive Chemistry/Solutions Is Copper Hydroxide Aqueous Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The nature of the resulting. The typical color of copper hydroxide is blue. If. Is Copper Hydroxide Aqueous.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Is Copper Hydroxide Aqueous Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The nature of the resulting. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The typical color of copper hydroxide is blue. Sat, 19 oct 2024 12:27:08 gmt simple The production of copper (ii) hydroxide typically occurs through the reaction of. Is Copper Hydroxide Aqueous.
From ar.inspiredpencil.com
Copper Hydroxide Solution Is Copper Hydroxide Aqueous The nature of the resulting. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has. Is Copper Hydroxide Aqueous.
From www.youtube.com
Make copper hydroxide with just two compounds YouTube Is Copper Hydroxide Aqueous The nature of the resulting. The typical color of copper hydroxide is blue. Sat, 19 oct 2024 12:27:08 gmt simple Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia is used instead of. Is Copper Hydroxide Aqueous.
From www.chegg.com
Solved 1. Aqueous ammonia solution and copper (II) nitrate Is Copper Hydroxide Aqueous The nature of the resulting. The typical color of copper hydroxide is blue. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Sat, 19 oct 2024 12:27:08 gmt simple Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant.. Is Copper Hydroxide Aqueous.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9449 Science Photo Is Copper Hydroxide Aqueous Sat, 19 oct 2024 12:27:08 gmt simple The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Copper (ii) hydroxide has some small solubility in water, determined. Is Copper Hydroxide Aqueous.
From woelen.homescience.net
Science made alive Chemistry/Solutions Is Copper Hydroxide Aqueous The nature of the resulting. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. The typical color of copper hydroxide is blue. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater. Is Copper Hydroxide Aqueous.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Is Copper Hydroxide Aqueous The nature of the resulting. Sat, 19 oct 2024 12:27:08 gmt simple The typical color of copper hydroxide is blue. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii). Is Copper Hydroxide Aqueous.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Is Copper Hydroxide Aqueous The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The nature of the resulting. The typical color of copper hydroxide is blue. Copper (ii) hydroxide has. Is Copper Hydroxide Aqueous.
From ablogwithadifference.com
Copper Hydroxide and Copper Oxychloride Best 5 difference Is Copper Hydroxide Aqueous The typical color of copper hydroxide is blue. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but. Is Copper Hydroxide Aqueous.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Is Copper Hydroxide Aqueous The typical color of copper hydroxide is blue. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Sat, 19 oct 2024 12:27:08 gmt simple The production of copper (ii). Is Copper Hydroxide Aqueous.
From flatworldknowledge.lardbucket.org
Electrochemistry Is Copper Hydroxide Aqueous Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The typical color of copper hydroxide is blue. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The nature of the resulting. Copper (ii) hydroxide has some small solubility in water,. Is Copper Hydroxide Aqueous.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Is Copper Hydroxide Aqueous Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Sat, 19 oct 2024 12:27:08 gmt simple The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. The nature of the resulting. If aqueous. Is Copper Hydroxide Aqueous.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + Cu(NO3)2 = NaNO3 + Cu(OH Is Copper Hydroxide Aqueous Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The nature of the resulting. The typical. Is Copper Hydroxide Aqueous.
From terracoreab.com.se
Hydroxides Terracoreab Is Copper Hydroxide Aqueous The nature of the resulting. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide. Is Copper Hydroxide Aqueous.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Is Copper Hydroxide Aqueous The nature of the resulting. The typical color of copper hydroxide is blue. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Sat, 19 oct 2024 12:27:08 gmt simple Copper(ii) hydroxide can be produced by adding sodium hydroxide. Is Copper Hydroxide Aqueous.
From www.sciencephoto.com
Copper hydroxide precipitate Stock Image A500/0411 Science Photo Is Copper Hydroxide Aqueous If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The typical color of copper hydroxide is blue. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal. Is Copper Hydroxide Aqueous.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9450 Science Photo Is Copper Hydroxide Aqueous Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide (chemical formula cu(oh) 2) is. Is Copper Hydroxide Aqueous.
From chemistry.com.pk
Colours of Transition Metal Ions in Aqueous Solution [Infographic Is Copper Hydroxide Aqueous The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. The typical color of copper hydroxide is blue. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The nature of the resulting. Sat, 19 oct 2024 12:27:08 gmt simple Copper(ii) hydroxide can be produced by adding sodium hydroxide. Is Copper Hydroxide Aqueous.
From woelen.homescience.net
Science made alive Chemistry/Solutions Is Copper Hydroxide Aqueous The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Copper(ii) hydroxide can be. Is Copper Hydroxide Aqueous.
From www.sciencephoto.com
Copper Hydroxide Precipitate, 3 of 3 Stock Image C030/8096 Is Copper Hydroxide Aqueous Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. The. Is Copper Hydroxide Aqueous.
From fineartamerica.com
Precipitation Of Copper (ii) Hydroxide Photograph by David Taylor Is Copper Hydroxide Aqueous Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The typical color of copper hydroxide is blue. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii). Is Copper Hydroxide Aqueous.
From woelen.homescience.net
Science made alive Chemistry/Solutions Is Copper Hydroxide Aqueous The nature of the resulting. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The typical color of. Is Copper Hydroxide Aqueous.
From www.youtube.com
QA Lab Practical Test for Cations Copper(II) ion, Cu2+ with aqueous Is Copper Hydroxide Aqueous Sat, 19 oct 2024 12:27:08 gmt simple The typical color of copper hydroxide is blue. The nature of the resulting. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide. Is Copper Hydroxide Aqueous.
From www.ceramic-glazes.com
Copper Hydroxide Cupric hydroxide patina for ceramics Is Copper Hydroxide Aqueous Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The typical color of copper hydroxide is blue. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Sat, 19 oct 2024 12:27:08 gmt simple Copper(ii) hydroxide can be produced by adding. Is Copper Hydroxide Aqueous.
From www.youtube.com
Making Copper Hydroxide YouTube Is Copper Hydroxide Aqueous The nature of the resulting. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The typical color of copper hydroxide is blue. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia. Is Copper Hydroxide Aqueous.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Is Copper Hydroxide Aqueous The nature of the resulting. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The typical color of copper hydroxide is blue. Sat, 19 oct 2024 12:27:08 gmt simple Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii). Is Copper Hydroxide Aqueous.
From www.alamy.com
Copper hydroxide on filter paper, flattened out. Copper (II) hydroxide Is Copper Hydroxide Aqueous Sat, 19 oct 2024 12:27:08 gmt simple The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. The nature of the resulting. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. The typical. Is Copper Hydroxide Aqueous.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Is Copper Hydroxide Aqueous The typical color of copper hydroxide is blue. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Sat, 19 oct 2024 12:27:08 gmt simple Copper(ii) hydroxide. Is Copper Hydroxide Aqueous.
From www.shutterstock.com
Copper Hydroxide Properties Chemical Compound Structure Stock Vector Is Copper Hydroxide Aqueous The typical color of copper hydroxide is blue. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The nature of the resulting. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. Copper(ii) hydroxide (chemical formula cu(oh) 2) is. Is Copper Hydroxide Aqueous.
From www.youtube.com
Reaction of Copper Sulphate (CuSO4) with Sodium Hydroxide (NaOH) YouTube Is Copper Hydroxide Aqueous If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The nature of the resulting. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an. Is Copper Hydroxide Aqueous.
From www.alamy.com
Copper hydroxide precipitate formed by adding sodium hydroxide (NaOH Is Copper Hydroxide Aqueous Sat, 19 oct 2024 12:27:08 gmt simple The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. The nature of the resulting. Copper (ii) hydroxide has some small solubility in water, determined by its solubility product constant. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much. Is Copper Hydroxide Aqueous.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Is Copper Hydroxide Aqueous Copper(ii) hydroxide (chemical formula cu(oh) 2) is the hydroxide of the metal copper. The production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. The typical color of. Is Copper Hydroxide Aqueous.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Is Copper Hydroxide Aqueous If aqueous ammonia is used instead of sodium hydroxide, the copper(ii) hydroxide precipitated has much greater air stability, but if excess ammonia is added, the. Sat, 19 oct 2024 12:27:08 gmt simple The typical color of copper hydroxide is blue. Copper(ii) hydroxide can be produced by adding sodium hydroxide to various copper(ii) sources. Copper (ii) hydroxide has some small solubility. Is Copper Hydroxide Aqueous.