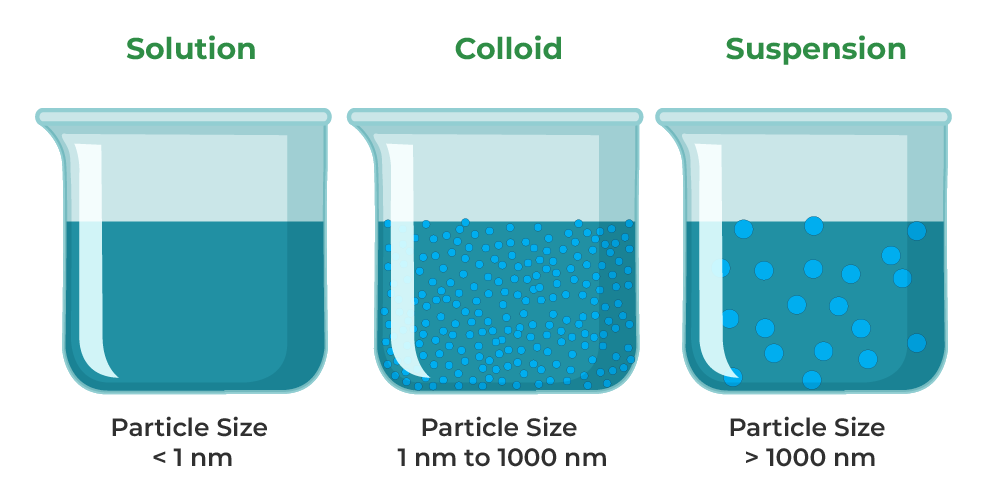
Suspension Colloid And Solution Examples . Examples examples solution of salt and water, sugar and. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is an intermediate between solution and suspension. Solution suspension colloid it is a homogeneous mixture it is a. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. A colloid is easily visible to the naked eye. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. Compare colloids, suspensions, solutions, and find their differences. All three are examples of colloids. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. It has particles with sizes between 2 and 1000 nanometers. Everyday examples of suspensions include mud, flour in water, and dust in air. Both suspensions and colloids are heterogeneous.
from www.geeksforgeeks.org
Solution suspension colloid it is a homogeneous mixture it is a. It has particles with sizes between 2 and 1000 nanometers. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. All three are examples of colloids. Compare colloids, suspensions, solutions, and find their differences. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. A colloid is an intermediate between solution and suspension. A colloid is easily visible to the naked eye. Everyday examples of suspensions include mud, flour in water, and dust in air. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash.
Suspension(Chemistry) Definition, Properties, Examples, and FAQs
Suspension Colloid And Solution Examples A colloid is an intermediate between solution and suspension. Examples examples solution of salt and water, sugar and. Compare colloids, suspensions, solutions, and find their differences. Solution suspension colloid it is a homogeneous mixture it is a. A colloid is easily visible to the naked eye. It has particles with sizes between 2 and 1000 nanometers. Both suspensions and colloids are heterogeneous. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. All three are examples of colloids. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. A colloid is an intermediate between solution and suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Everyday examples of suspensions include mud, flour in water, and dust in air. Understand particles in a solution, and explore solution, suspension, and colloid examples.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Colloid And Solution Examples Everyday examples of suspensions include mud, flour in water, and dust in air. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. Compare colloids, suspensions, solutions, and find their differences.. Suspension Colloid And Solution Examples.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free download ID289762 Suspension Colloid And Solution Examples Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. A colloid is easily visible to the naked eye. Examples examples solution of salt and water, sugar and. Day to day. Suspension Colloid And Solution Examples.
From www.youtube.com
SOLUTION, SUSPENSION AND COLLOID MIXTURES CHEMISTRY 720p YouTube Suspension Colloid And Solution Examples Examples examples solution of salt and water, sugar and. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is easily visible to the naked eye. Both suspensions and colloids are heterogeneous. Compare colloids, suspensions, solutions, and find their differences. Everyday examples of suspensions include mud, flour in water, and dust in air. Suspensions and colloids. Suspension Colloid And Solution Examples.
From www.youtube.com
Science 6 Q1 Week 2 Solution, Suspension, Colloid YouTube Suspension Colloid And Solution Examples It has particles with sizes between 2 and 1000 nanometers. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. A colloid is easily visible to the naked eye. Everyday examples of suspensions include mud, flour in water, and dust in air. Understand particles. Suspension Colloid And Solution Examples.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of solution 21669338 Vector Suspension Colloid And Solution Examples A colloid is easily visible to the naked eye. Everyday examples of suspensions include mud, flour in water, and dust in air. Understand particles in a solution, and explore solution, suspension, and colloid examples. Both suspensions and colloids are heterogeneous. Solution suspension colloid it is a homogeneous mixture it is a. Suspensions and colloids are two common types of mixtures. Suspension Colloid And Solution Examples.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram, Science lessons Suspension Colloid And Solution Examples Examples examples solution of salt and water, sugar and. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. It has particles with sizes between 2 and 1000 nanometers. A colloid is an intermediate between solution and suspension. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate. Suspension Colloid And Solution Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid And Solution Examples Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. All three are examples of colloids. It has particles with sizes between 2 and 1000 nanometers. Compare colloids, suspensions, solutions, and find their differences. Day to day examples like milk which is considered to be the best. Suspension Colloid And Solution Examples.
From www.vecteezy.com
Different dispersion system, true and colloidal solution and suspension based on the size of Suspension Colloid And Solution Examples Everyday examples of suspensions include mud, flour in water, and dust in air. Compare colloids, suspensions, solutions, and find their differences. A colloid is easily visible to the naked eye. Examples examples solution of salt and water, sugar and. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is an intermediate between solution and suspension.. Suspension Colloid And Solution Examples.
From pt.slideshare.net
Solutions, suspensions, and colloids Suspension Colloid And Solution Examples Everyday examples of suspensions include mud, flour in water, and dust in air. A colloid is an intermediate between solution and suspension. Both suspensions and colloids are heterogeneous. Understand particles in a solution, and explore solution, suspension, and colloid examples. Solution suspension colloid it is a homogeneous mixture it is a. A colloid is easily visible to the naked eye.. Suspension Colloid And Solution Examples.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free download ID1066087 Suspension Colloid And Solution Examples Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is an intermediate between solution and suspension. It has particles with sizes between 2 and 1000 nanometers. A colloid is. Suspension Colloid And Solution Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid And Solution Examples All three are examples of colloids. It has particles with sizes between 2 and 1000 nanometers. Compare colloids, suspensions, solutions, and find their differences. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Suspensions may scatter light, but if the number of suspended particles is sufficiently. Suspension Colloid And Solution Examples.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Colloid And Solution Examples Compare colloids, suspensions, solutions, and find their differences. Both suspensions and colloids are heterogeneous. A colloid is easily visible to the naked eye. All three are examples of colloids. Examples examples solution of salt and water, sugar and. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is an intermediate between solution and suspension. It. Suspension Colloid And Solution Examples.
From sciencenotes.org
What Is a Colloid? Definition and Examples Suspension Colloid And Solution Examples Examples examples solution of salt and water, sugar and. All three are examples of colloids. Everyday examples of suspensions include mud, flour in water, and dust in air. Compare colloids, suspensions, solutions, and find their differences. Understand particles in a solution, and explore solution, suspension, and colloid examples. Both suspensions and colloids are heterogeneous. A colloid is easily visible to. Suspension Colloid And Solution Examples.
From www.youtube.com
Solution Suspension Colloid YouTube Suspension Colloid And Solution Examples Everyday examples of suspensions include mud, flour in water, and dust in air. Compare colloids, suspensions, solutions, and find their differences. It has particles with sizes between 2 and 1000 nanometers. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. All three are examples of colloids.. Suspension Colloid And Solution Examples.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Surface Chemistry Suspension Colloid And Solution Examples Both suspensions and colloids are heterogeneous. Compare colloids, suspensions, solutions, and find their differences. Everyday examples of suspensions include mud, flour in water, and dust in air. Solution suspension colloid it is a homogeneous mixture it is a. All three are examples of colloids. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension.. Suspension Colloid And Solution Examples.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID1292191 Suspension Colloid And Solution Examples Compare colloids, suspensions, solutions, and find their differences. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. All three are examples of colloids. It has particles with sizes between 2 and 1000 nanometers. A colloid is easily visible to the naked eye. Solution suspension colloid it. Suspension Colloid And Solution Examples.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Suspension Colloid And Solution Examples Solution suspension colloid it is a homogeneous mixture it is a. Both suspensions and colloids are heterogeneous. Understand particles in a solution, and explore solution, suspension, and colloid examples. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. All three are examples of colloids. Suspensions and colloids are two common types of mixtures. Suspension Colloid And Solution Examples.
From www.pinterest.com
mixture suspension colloid Google Search Chemistry worksheets, Science lessons, Science Suspension Colloid And Solution Examples A colloid is easily visible to the naked eye. Understand particles in a solution, and explore solution, suspension, and colloid examples. It has particles with sizes between 2 and 1000 nanometers. Examples examples solution of salt and water, sugar and. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous. Suspension Colloid And Solution Examples.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Colloid And Solution Examples A colloid is easily visible to the naked eye. Solution suspension colloid it is a homogeneous mixture it is a. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Everyday examples of suspensions include mud, flour in water, and dust in air. Day to day examples. Suspension Colloid And Solution Examples.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Suspension Colloid And Solution Examples It has particles with sizes between 2 and 1000 nanometers. Examples examples solution of salt and water, sugar and. Solution suspension colloid it is a homogeneous mixture it is a. Everyday examples of suspensions include mud, flour in water, and dust in air. Day to day examples like milk which is considered to be the best example of colloid, the. Suspension Colloid And Solution Examples.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Chemistry Aakash Suspension Colloid And Solution Examples Both suspensions and colloids are heterogeneous. A colloid is an intermediate between solution and suspension. A colloid is easily visible to the naked eye. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Suspensions and colloids are two common types of mixtures whose. Suspension Colloid And Solution Examples.
From www.sliderbase.com
Suspensions and Colloids Presentation Chemistry Suspension Colloid And Solution Examples Examples examples solution of salt and water, sugar and. Both suspensions and colloids are heterogeneous. All three are examples of colloids. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension.. Suspension Colloid And Solution Examples.
From slidetodoc.com
UNDERSTANDING SOLUTIONS PART 1 SOLUTIONS SUSPENSIONS A suspension Suspension Colloid And Solution Examples It has particles with sizes between 2 and 1000 nanometers. Solution suspension colloid it is a homogeneous mixture it is a. A colloid is easily visible to the naked eye. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. A colloid is an. Suspension Colloid And Solution Examples.
From www.slideshare.net
Solutions, suspensions, and colloids Suspension Colloid And Solution Examples Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Solution suspension colloid it is a homogeneous mixture it is a. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. Everyday examples of suspensions include mud, flour. Suspension Colloid And Solution Examples.
From chemistry-examples-00.blogspot.com
8 EXAMPLES OF SUSPENSION COLLOID AND SOLUTION, SUSPENSION OF SOLUTION AND EXAMPLES COLLOID Suspension Colloid And Solution Examples It has particles with sizes between 2 and 1000 nanometers. Both suspensions and colloids are heterogeneous. A colloid is easily visible to the naked eye. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Everyday examples of suspensions include mud, flour in water,. Suspension Colloid And Solution Examples.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Suspension Colloid And Solution Examples It has particles with sizes between 2 and 1000 nanometers. A colloid is easily visible to the naked eye. Solution suspension colloid it is a homogeneous mixture it is a. Compare colloids, suspensions, solutions, and find their differences. All three are examples of colloids. Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate. Suspension Colloid And Solution Examples.
From www.youtube.com
Solution, Suspension and Colloid aumsum kids education science learn YouTube Suspension Colloid And Solution Examples A colloid is easily visible to the naked eye. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Examples examples solution of salt and water, sugar and. Understand particles in a solution, and explore solution, suspension, and colloid examples. Suspensions and colloids are. Suspension Colloid And Solution Examples.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download ID822807 Suspension Colloid And Solution Examples Compare colloids, suspensions, solutions, and find their differences. Everyday examples of suspensions include mud, flour in water, and dust in air. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Examples examples solution of salt and water, sugar and. Solution suspension colloid it. Suspension Colloid And Solution Examples.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download ID6666151 Suspension Colloid And Solution Examples Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Everyday examples of suspensions include mud, flour in water, and dust in air. All three are examples of colloids. Examples examples solution of salt and water, sugar and. It has particles with sizes between 2 and 1000. Suspension Colloid And Solution Examples.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension Colloid And Solution Examples It has particles with sizes between 2 and 1000 nanometers. Both suspensions and colloids are heterogeneous. Everyday examples of suspensions include mud, flour in water, and dust in air. A colloid is an intermediate between solution and suspension. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. Compare colloids, suspensions, solutions, and find. Suspension Colloid And Solution Examples.
From www.slideserve.com
PPT Mixtures PowerPoint Presentation, free download ID3086482 Suspension Colloid And Solution Examples Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. It has particles with sizes between 2 and 1000 nanometers. Understand particles in a solution, and explore solution, suspension, and colloid examples. Both suspensions and colloids are heterogeneous. Day to day examples like milk which is considered. Suspension Colloid And Solution Examples.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True solutions Colloids Suspensions Suspension Colloid And Solution Examples Solution suspension colloid it is a homogeneous mixture it is a. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. Examples examples solution of salt and water,. Suspension Colloid And Solution Examples.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Suspension Colloid And Solution Examples A colloid is an intermediate between solution and suspension. A colloid is easily visible to the naked eye. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. Both suspensions and colloids are heterogeneous. Everyday examples of suspensions include mud, flour in water, and dust in air. All three are examples of colloids. Solution. Suspension Colloid And Solution Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Colloid And Solution Examples Both suspensions and colloids are heterogeneous. Compare colloids, suspensions, solutions, and find their differences. Suspensions may scatter light, but if the number of suspended particles is sufficiently large, the suspension. It has particles with sizes between 2 and 1000 nanometers. A colloid is an intermediate between solution and suspension. Examples examples solution of salt and water, sugar and. A colloid. Suspension Colloid And Solution Examples.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Suspension Colloid And Solution Examples Suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and heterogeneous mixtures. Day to day examples like milk which is considered to be the best example of colloid, the shampoo that we get to use, liquid hand wash. Everyday examples of suspensions include mud, flour in water, and dust. Suspension Colloid And Solution Examples.