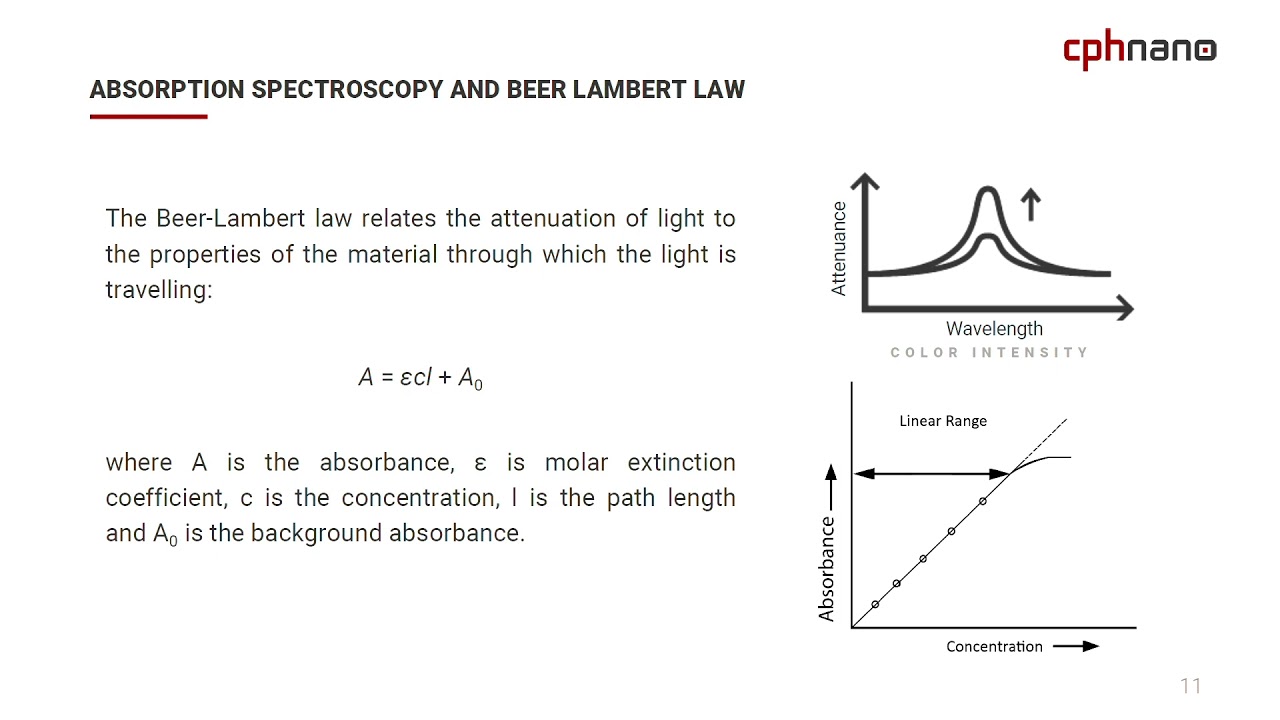
What Is The Beer Lambert Law Used For . Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless.
from www.youtube.com
\[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
Absorption spectroscopy (BeerLambert law) presented by PhD Emil
What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless.
From www.toppr.com
Beer Lambert Law Statement, Derivation, Formula and Questions What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From chemistrytalk.org
BeerLambert Law ChemTalk What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From chemistrytalk.org
BeerLambert Law ChemTalk What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From www.examples.com
BeerLambert Law Definition, Formula,Examples,Limitations What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From biologynotesonline.com
BeerLambert Law Definition, Derivation, and Limitations Biology What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.learnatnoon.com
What are some Limitations of Beer Lambert Law? Noon Academy What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From sciencenotes.org
Beer's Law Equation and Example What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.youtube.com
Beer's Law Overview YouTube What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.slideshare.net
Beer lambert Law What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From www.youtube.com
Beer Lambert law derivation and usage YouTube What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.youtube.com
Derivation of Beer Lambert Law YouTube What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.youtube.com
BeerLambert law in easy way YouTube What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.thoughtco.com
Beer's Law Definition and Equation What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From www.youtube.com
spectrum, UV Visible Spectroscopy, Beer Lambert’s Law What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From mungfali.com
UV Spectroscopy Beer Lambert Law What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube What Is The Beer Lambert Law Used For Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. What Is The Beer Lambert Law Used For.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. What Is The Beer Lambert Law Used For.
From www.youtube.com
Spectrophotometry BeerLambert Law. YouTube What Is The Beer Lambert Law Used For \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.
From www.youtube.com
Beer and Lambert Law Derivation YouTube What Is The Beer Lambert Law Used For Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. What Is The Beer Lambert Law Used For.