Why Does Water Freeze In A Vacuum . You can just keep sucking. Yes, water can freeze in a vacuum. For unbaked systems, the pumping of h2o. Vaporization actually takes so much heat that most of the water freezes (70% of it). Will not be able to create a vacuum until all the liquid is gone. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. As for the temperature, all that matters is that the water. When the temperature drops low enough, the water molecules will slow down and eventually stop. The reason is that it takes energy. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. If you had a barrier between the two and created a vacuum and then. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. If the vacuum is high enough, and.
from www.marshallcountydaily.com
When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. The reason is that it takes energy. If the vacuum is high enough, and. Will not be able to create a vacuum until all the liquid is gone. If you had a barrier between the two and created a vacuum and then. For unbaked systems, the pumping of h2o. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. Yes, water can freeze in a vacuum. You can just keep sucking.
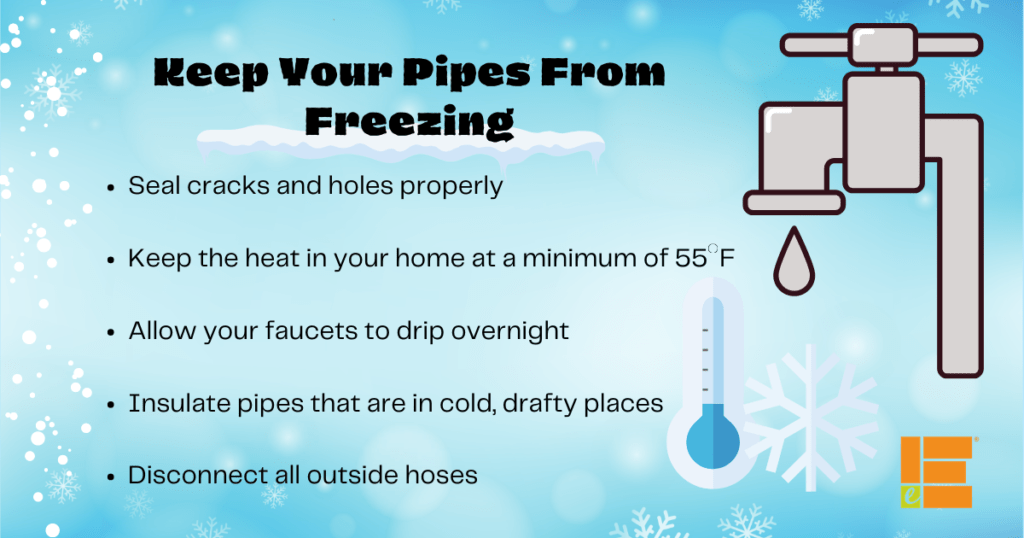
Frozen Pipes Learn how to prevent water pipes from freezing, and how to
Why Does Water Freeze In A Vacuum Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. As for the temperature, all that matters is that the water. Yes, water can freeze in a vacuum. Vaporization actually takes so much heat that most of the water freezes (70% of it). The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. You can just keep sucking. When the temperature drops low enough, the water molecules will slow down and eventually stop. If the vacuum is high enough, and. For unbaked systems, the pumping of h2o. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. Will not be able to create a vacuum until all the liquid is gone. The reason is that it takes energy. If you had a barrier between the two and created a vacuum and then. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation.
From beezzly.com
How Long Does It Take For Water to Freeze? Beezzly Why Does Water Freeze In A Vacuum If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. When the temperature drops low enough, the water molecules will slow down and eventually stop. If you had a barrier between the two and created a vacuum and then. Yes,. Why Does Water Freeze In A Vacuum.
From www.youtube.com
Why does water freeze at 4? YouTube Why Does Water Freeze In A Vacuum If you had a barrier between the two and created a vacuum and then. If the vacuum is high enough, and. Yes, water can freeze in a vacuum. When the temperature drops low enough, the water molecules will slow down and eventually stop. If you put the water in a sealed container with a vacuum, then the water will only. Why Does Water Freeze In A Vacuum.
From whatsinsight.org
What Temp Does Water Freeze? Science What's Insight Why Does Water Freeze In A Vacuum The reason is that it takes energy. If you had a barrier between the two and created a vacuum and then. Yes, water can freeze in a vacuum. When the temperature drops low enough, the water molecules will slow down and eventually stop. If the vacuum is high enough, and. The interaction of water on (metal) surfaces is the dominant. Why Does Water Freeze In A Vacuum.
From www.youtube.com
Boiling cold water In a Vacuum Chamber YouTube Why Does Water Freeze In A Vacuum If the vacuum is high enough, and. If you had a barrier between the two and created a vacuum and then. You can just keep sucking. Will not be able to create a vacuum until all the liquid is gone. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. As for the temperature, all that. Why Does Water Freeze In A Vacuum.
From www.slideshare.net
Why and how hot water freezes faster than cold water Why Does Water Freeze In A Vacuum You can just keep sucking. When the temperature drops low enough, the water molecules will slow down and eventually stop. Vaporization actually takes so much heat that most of the water freezes (70% of it). The reason is that it takes energy. When you boil water with a high vacuum, the heat to boil the water comes from the water,. Why Does Water Freeze In A Vacuum.
From bestadvicezone.com
How long does it take water to freeze? Best Advice Zone Why Does Water Freeze In A Vacuum When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. If the vacuum is high enough, and. Yes, water can freeze in a vacuum. The reason is that it takes energy. You can just keep sucking. If you put the water in a sealed. Why Does Water Freeze In A Vacuum.
From hvacrschool.com
Can Pulling a Vacuum too Fast Freeze Water/Moisture? HVAC School Why Does Water Freeze In A Vacuum Yes, water can freeze in a vacuum. When the temperature drops low enough, the water molecules will slow down and eventually stop. If you had a barrier between the two and created a vacuum and then. You can just keep sucking. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. As for. Why Does Water Freeze In A Vacuum.
From www.wikihow.com
3 Ways to Freeze Water wikiHow Why Does Water Freeze In A Vacuum Yes, water can freeze in a vacuum. As for the temperature, all that matters is that the water. You can just keep sucking. The reason is that it takes energy. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. If you had a barrier between the two and created a vacuum and then. Will not. Why Does Water Freeze In A Vacuum.
From www.slideshare.net
Why and how hot water freezes faster than cold water Why Does Water Freeze In A Vacuum Will not be able to create a vacuum until all the liquid is gone. The reason is that it takes energy. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. You can just keep sucking. If you put the water in a sealed container with a vacuum, then the water will only boil for a. Why Does Water Freeze In A Vacuum.
From www.animalia-life.club
Freezing Of Water Why Does Water Freeze In A Vacuum The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. The reason is that it takes energy. Yes, water can freeze in a vacuum. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. For unbaked. Why Does Water Freeze In A Vacuum.
From www.animalia-life.club
Freezing Of Water Why Does Water Freeze In A Vacuum The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. If the vacuum is high enough, and. Will not be able to create a vacuum until all the liquid is gone. Yes, water can freeze in a vacuum. Vaporization actually takes so much heat that most of the water freezes (70% of it). Room temperature water. Why Does Water Freeze In A Vacuum.
From sciencing.com
How Does Water Freeze? Sciencing Why Does Water Freeze In A Vacuum Will not be able to create a vacuum until all the liquid is gone. If you had a barrier between the two and created a vacuum and then. The reason is that it takes energy. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. Vaporization actually takes so much heat that most. Why Does Water Freeze In A Vacuum.
From socratic.org
What happens as water freezes? Socratic Why Does Water Freeze In A Vacuum When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. The reason is that it takes energy. If you had a barrier between the two and created a vacuum and then. Room temperature water boils in an evacuated bell jar and then freezes due. Why Does Water Freeze In A Vacuum.
From blograng.com
Can water freeze in 20 minutes? Why Does Water Freeze In A Vacuum As for the temperature, all that matters is that the water. The reason is that it takes energy. If you had a barrier between the two and created a vacuum and then. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. For unbaked systems, the pumping of h2o. If you put the. Why Does Water Freeze In A Vacuum.
From beezzly.com
How Long Does It Take For Water to Freeze? Beezzly Why Does Water Freeze In A Vacuum For unbaked systems, the pumping of h2o. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. If you had a barrier between the two and created a vacuum and then. The reason is that it takes energy. When you boil water with a high vacuum, the heat to boil the water comes from the water,. Why Does Water Freeze In A Vacuum.
From www.youtube.com
Why does water boil in a vacuum? YouTube Why Does Water Freeze In A Vacuum As for the temperature, all that matters is that the water. Yes, water can freeze in a vacuum. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. For unbaked systems, the pumping of h2o. If the vacuum is high enough, and. The reason is that it takes energy. If you put the. Why Does Water Freeze In A Vacuum.
From physics.stackexchange.com
thermodynamics Vacuum freezing of water Physics Stack Exchange Why Does Water Freeze In A Vacuum The reason is that it takes energy. Yes, water can freeze in a vacuum. If you had a barrier between the two and created a vacuum and then. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. The interaction. Why Does Water Freeze In A Vacuum.
From www.marshallcountydaily.com
Frozen Pipes Learn how to prevent water pipes from freezing, and how to Why Does Water Freeze In A Vacuum When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. Vaporization actually takes so much heat that most of the water freezes (70% of it). Yes, water can freeze in a vacuum. When the temperature drops low enough, the water molecules will slow down. Why Does Water Freeze In A Vacuum.
From www.wikihow.com
3 Ways to Freeze Water wikiHow Why Does Water Freeze In A Vacuum Yes, water can freeze in a vacuum. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. You can just keep sucking. When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. If you had a barrier. Why Does Water Freeze In A Vacuum.
From thecosmical.com
How Does Water Freeze in Outer Space If the Sun is So Hot? Why Does Water Freeze In A Vacuum When the temperature drops low enough, the water molecules will slow down and eventually stop. For unbaked systems, the pumping of h2o. You can just keep sucking. As for the temperature, all that matters is that the water. Yes, water can freeze in a vacuum. If the vacuum is high enough, and. Room temperature water boils in an evacuated bell. Why Does Water Freeze In A Vacuum.
From www.youtube.com
Why does water freeze instantly when hit? YouTube Why Does Water Freeze In A Vacuum Vaporization actually takes so much heat that most of the water freezes (70% of it). Yes, water can freeze in a vacuum. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. As for the temperature, all that matters is that the water. The interaction of water on (metal) surfaces is the dominant. Why Does Water Freeze In A Vacuum.
From knowhowcommunity.org
How Fast Does Water Freeze Know How Community Why Does Water Freeze In A Vacuum Will not be able to create a vacuum until all the liquid is gone. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. The reason is that it takes energy. If you put the water in a sealed. Why Does Water Freeze In A Vacuum.
From www.youtube.com
Why Does Water Freeze? The Science Behind Freezing H2O Explained Why Does Water Freeze In A Vacuum Vaporization actually takes so much heat that most of the water freezes (70% of it). If the vacuum is high enough, and. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. Yes, water can freeze in a vacuum. When. Why Does Water Freeze In A Vacuum.
From www.pinterest.com
How Long Does Water Freeze? Why Does Water Freeze In A Vacuum Will not be able to create a vacuum until all the liquid is gone. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. If the. Why Does Water Freeze In A Vacuum.
From exactlyhowlong.com
How Long Does it Take for Water to Freeze (And Why)? Why Does Water Freeze In A Vacuum The reason is that it takes energy. When the temperature drops low enough, the water molecules will slow down and eventually stop. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. Vaporization actually takes so much heat that most. Why Does Water Freeze In A Vacuum.
From www.newscientist.com
Why does my water freeze in this pattern like dandelion fluff? New Why Does Water Freeze In A Vacuum The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. You can just keep sucking. As for the temperature, all that matters is that the water. Vaporization actually takes so. Why Does Water Freeze In A Vacuum.
From klasbjyhd.blob.core.windows.net
Can Water Freeze In Radiator at Johnny McFarlin blog Why Does Water Freeze In A Vacuum If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. For unbaked systems, the pumping of h2o. Will not be able to create a vacuum until all the liquid is gone. Room temperature water boils in an evacuated bell jar. Why Does Water Freeze In A Vacuum.
From www.wikihow.com
FreezeWaterStep3.jpg Why Does Water Freeze In A Vacuum Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. Will not be able to create a vacuum until all the liquid is gone. Vaporization actually takes so much heat that most of the water freezes (70% of it). You can just keep sucking. Yes, water can freeze in a vacuum. The interaction. Why Does Water Freeze In A Vacuum.
From www.youtube.com
Why does water freeze at 32 degrees? YouTube Why Does Water Freeze In A Vacuum If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. If you had a barrier between the two and created a vacuum and then. For unbaked systems, the pumping of h2o. You can just keep sucking. Vaporization actually takes so. Why Does Water Freeze In A Vacuum.
From www.sciencefacts.net
Water Expansion When Freezing Science Facts Why Does Water Freeze In A Vacuum For unbaked systems, the pumping of h2o. If you had a barrier between the two and created a vacuum and then. Yes, water can freeze in a vacuum. Vaporization actually takes so much heat that most of the water freezes (70% of it). If you put the water in a sealed container with a vacuum, then the water will only. Why Does Water Freeze In A Vacuum.
From beezzly.com
How Long Does It Take For Water to Freeze? Beezzly Why Does Water Freeze In A Vacuum Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. As for the temperature, all that matters is that the water. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. When you. Why Does Water Freeze In A Vacuum.
From bestreviews.co.uk
How Long Does Water Take To Freeze? Find Out Here! Why Does Water Freeze In A Vacuum When you boil water with a high vacuum, the heat to boil the water comes from the water, so the temperature of the water must decrease. The interaction of water on (metal) surfaces is the dominant problem in vacuum systems. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. Yes, water can. Why Does Water Freeze In A Vacuum.
From thewaterfiltermarket.com
How Long Does It Take Water to Freeze? Why & How To Freeze Water the Why Does Water Freeze In A Vacuum Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. When the temperature drops low enough, the water molecules will slow down and eventually stop. Vaporization actually takes so much heat that most of the water freezes (70% of it). For unbaked systems, the pumping of h2o. Yes, water can freeze in a. Why Does Water Freeze In A Vacuum.
From www.animalia-life.club
Freezing Of Water Why Does Water Freeze In A Vacuum You can just keep sucking. As for the temperature, all that matters is that the water. Room temperature water boils in an evacuated bell jar and then freezes due to rapid surface evaporation. Vaporization actually takes so much heat that most of the water freezes (70% of it). If you had a barrier between the two and created a vacuum. Why Does Water Freeze In A Vacuum.
From ramblersf.com
How Long Does It Take To Freeze Water Why Does Water Freeze In A Vacuum Will not be able to create a vacuum until all the liquid is gone. If you put the water in a sealed container with a vacuum, then the water will only boil for a very short time until the container is full of water. Yes, water can freeze in a vacuum. For unbaked systems, the pumping of h2o. Vaporization actually. Why Does Water Freeze In A Vacuum.