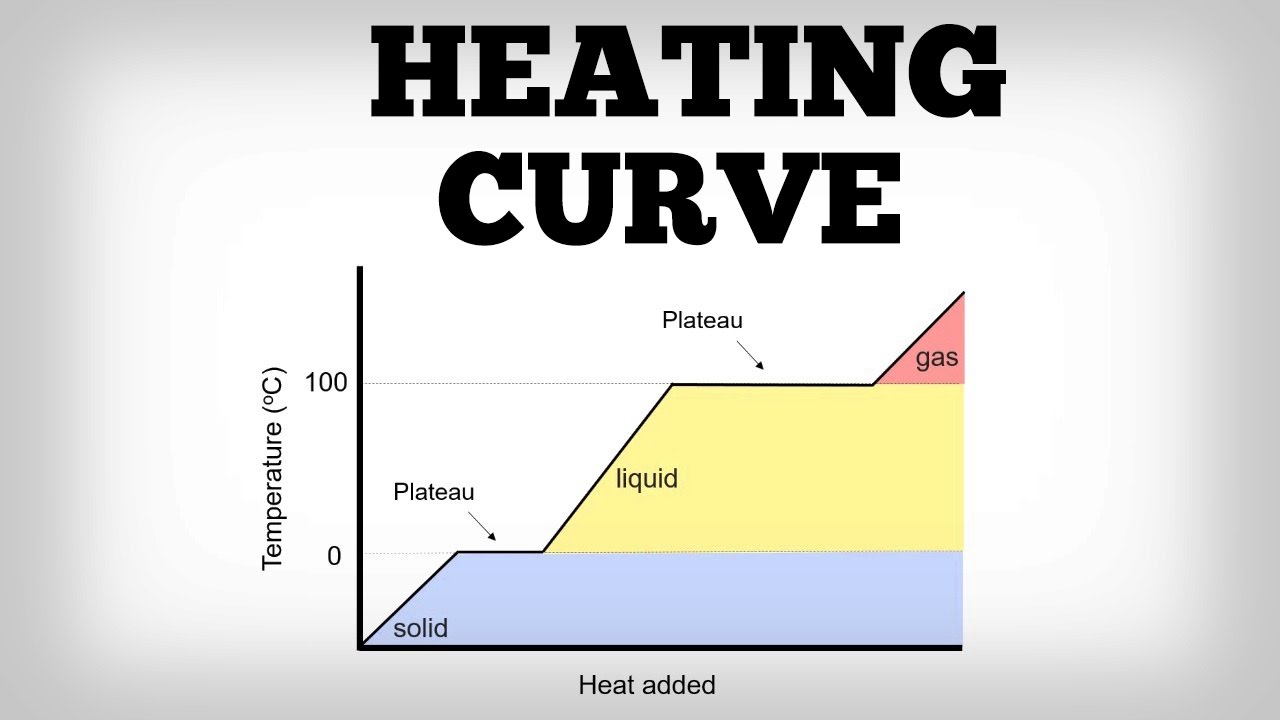
Heating Curve Solid To Gas . To change the state of a substance energy must be transferred to, or from, the. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Heating curves relate temperature changes to phase. Freezing is the opposite of melting, and both. Initially the system is a solid, then it has a melting. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Eventually, the pressure and temperature.
from www.youtube.com
During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Initially the system is a solid, then it has a melting. To change the state of a substance energy must be transferred to, or from, the. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Eventually, the pressure and temperature. Freezing is the opposite of melting, and both. Heating curves relate temperature changes to phase. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below.
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV
Heating Curve Solid To Gas Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Freezing is the opposite of melting, and both. Heating curves relate temperature changes to phase. Eventually, the pressure and temperature. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. To change the state of a substance energy must be transferred to, or from, the. Initially the system is a solid, then it has a melting. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Heating Curve Solid To Gas Freezing is the opposite of melting, and both. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. All of the changes of state. Heating Curve Solid To Gas.
From www.slideserve.com
PPT Molecular Theory States of Matter Phase Changes Heating Curve Solid To Gas Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Initially the system is a solid, then it has a melting. Eventually, the pressure and temperature. This can be easily seen in a heating curve that plots the temperature of a system as a function of. Heating Curve Solid To Gas.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Heating Curve Solid To Gas To change the state of a substance energy must be transferred to, or from, the. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in. Heating Curve Solid To Gas.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating Curve Solid To Gas This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Freezing is the opposite of melting, and both. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. During heating, the substance. Heating Curve Solid To Gas.
From preparatorychemistry.com
Heating Curve Heating Curve Solid To Gas Eventually, the pressure and temperature. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. To change the state of a substance energy must be transferred to, or from, the. Plots of the temperature of a substance versus heat added or versus heating time. Heating Curve Solid To Gas.
From www.showme.com
Heating and Cooling Curves Explained Science, Heating Curve, Cooling Heating Curve Solid To Gas This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Eventually, the pressure and temperature. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Heating curves relate temperature changes to phase.. Heating Curve Solid To Gas.
From slideplayer.com
Ch. 8 Solids, Liquids, & Gases ppt download Heating Curve Solid To Gas This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. During heating, the substance undergoes different phase transitions, such as solid to. Heating Curve Solid To Gas.
From watercoolingsengihi.blogspot.com
Water Cooling Water Cooling Curve Heating Curve Solid To Gas Initially the system is a solid, then it has a melting. Heating curves relate temperature changes to phase. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization),. Heating Curve Solid To Gas.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Solid To Gas All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Heating curves relate temperature changes to phase. Initially the system is a solid, then it has a melting. To change the state of a substance energy must be transferred to, or from, the. Plots of the temperature of. Heating Curve Solid To Gas.
From www.chegg.com
Solved Consider The Heating Curve For 1 Mole Of An Unknow... Heating Curve Solid To Gas Eventually, the pressure and temperature. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. During heating, the substance undergoes. Heating Curve Solid To Gas.
From mmerevise.co.uk
Specific Latent Heat Questions and Revision MME Heating Curve Solid To Gas Eventually, the pressure and temperature. Freezing is the opposite of melting, and both. Initially the system is a solid, then it has a melting. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. To change the state of a substance energy must be transferred to, or from,. Heating Curve Solid To Gas.
From ch301.cm.utexas.edu
heating curve Heating Curve Solid To Gas This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Heating curves relate temperature changes to phase. Freezing is the opposite of melting, and both. Initially the system is a solid, then it has a melting. Eventually, the pressure and temperature. All of the. Heating Curve Solid To Gas.
From quizizz.com
Heating and Cooling Curves Chemistry Quiz Quizizz Heating Curve Solid To Gas Initially the system is a solid, then it has a melting. To change the state of a substance energy must be transferred to, or from, the. Eventually, the pressure and temperature. Freezing is the opposite of melting, and both. This can be easily seen in a heating curve that plots the temperature of a system as a function of the. Heating Curve Solid To Gas.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk Heating Curve Solid To Gas During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Freezing is the opposite of melting, and both. Eventually, the pressure and temperature. To change the state of a substance energy must be transferred to, or from, the. Initially the system is a solid, then it has a. Heating Curve Solid To Gas.
From slideplayer.com
Heating Curves Ice melts by absorbing heat, but without increasing Heating Curve Solid To Gas All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Initially the system is a solid, then it has a melting. Eventually, the pressure and temperature. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat. Heating Curve Solid To Gas.
From www.chegg.com
Solved For the following heating curve, give the correct Heating Curve Solid To Gas Eventually, the pressure and temperature. Initially the system is a solid, then it has a melting. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas.. Heating Curve Solid To Gas.
From www.docbrown.info
GASES LIQUIDS SOLIDS States of Matter, particle theory models Heating Curve Solid To Gas Freezing is the opposite of melting, and both. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. To change the state of a substance energy must be transferred to, or from, the. Eventually, the pressure and temperature. Plots of the temperature of a. Heating Curve Solid To Gas.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve Solid To Gas Eventually, the pressure and temperature. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. To change the state of a substance energy must be transferred. Heating Curve Solid To Gas.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curve Solid To Gas During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Heating curves relate temperature changes to phase. Eventually, the pressure and temperature. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Initially the. Heating Curve Solid To Gas.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve Solid To Gas All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. To change the state of a substance energy must be transferred to, or from, the. Heating. Heating Curve Solid To Gas.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Solid To Gas To change the state of a substance energy must be transferred to, or from, the. Freezing is the opposite of melting, and both. Heating curves relate temperature changes to phase. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Eventually, the pressure and temperature. During heating, the. Heating Curve Solid To Gas.
From www.chegg.com
Solved Use the heating curve provided to answer the Heating Curve Solid To Gas This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Eventually, the pressure and temperature. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. To change the state of a substance. Heating Curve Solid To Gas.
From www.chegg.com
Solved Consider the heating curve of a substance in the Heating Curve Solid To Gas During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Plots of the temperature of a substance versus heat added or versus heating time at a. Heating Curve Solid To Gas.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Solid To Gas Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. Eventually, the pressure and temperature. All of the changes of. Heating Curve Solid To Gas.
From quizlet.com
Heating Curve Diagram Quizlet Heating Curve Solid To Gas Heating curves relate temperature changes to phase. To change the state of a substance energy must be transferred to, or from, the. Initially the system is a solid, then it has a melting. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Plots of the temperature of. Heating Curve Solid To Gas.
From brainly.com
Consider the heating curve provided for 1.00 mole of a substance that Heating Curve Solid To Gas Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. To change the state of a substance energy must be transferred to, or from, the. Initially the system is a solid, then it has a melting. Heating curves relate temperature changes to phase. Eventually, the pressure. Heating Curve Solid To Gas.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation Heating Curve Solid To Gas Initially the system is a solid, then it has a melting. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Eventually, the pressure and temperature. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat. Heating Curve Solid To Gas.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free Heating Curve Solid To Gas To change the state of a substance energy must be transferred to, or from, the. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Eventually, the pressure and temperature. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate. Heating Curve Solid To Gas.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Solid To Gas Heating curves relate temperature changes to phase. Initially the system is a solid, then it has a melting. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Eventually, the pressure and temperature. Plots of the temperature of a substance versus heat added or versus heating time at. Heating Curve Solid To Gas.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Solid To Gas This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Plots of the temperature of a substance versus heat added or versus. Heating Curve Solid To Gas.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Solid To Gas Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating curves relate temperature changes to phase. This can be easily seen in a heating curve that plots the temperature of a system as a function of the heat flow into the system. To change the. Heating Curve Solid To Gas.
From www.chegg.com
Solved Refer to the heating curve below. On the basis Heating Curve Solid To Gas To change the state of a substance energy must be transferred to, or from, the. Heating curves relate temperature changes to phase. All of the changes of state that occur between solid, liquid, and gas are summarized in the diagram in the figure below. Freezing is the opposite of melting, and both. This can be easily seen in a heating. Heating Curve Solid To Gas.
From www.coursehero.com
[Solved] Draw the heating curve for Zinc as it is heated from solid to Heating Curve Solid To Gas Heating curves relate temperature changes to phase. During heating, the substance undergoes different phase transitions, such as solid to liquid (melting), liquid to gas (vaporization), or solid to gas. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. This can be easily seen in a. Heating Curve Solid To Gas.
From www.doubtnut.com
The heating curve of a particular substance in solid state is a shown Heating Curve Solid To Gas To change the state of a substance energy must be transferred to, or from, the. Initially the system is a solid, then it has a melting. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. All of the changes of state that occur between solid,. Heating Curve Solid To Gas.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Solid To Gas Freezing is the opposite of melting, and both. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called heating curves. Heating curves relate temperature changes to phase. Initially the system is a solid, then it has a melting. This can be easily seen in a heating curve that. Heating Curve Solid To Gas.