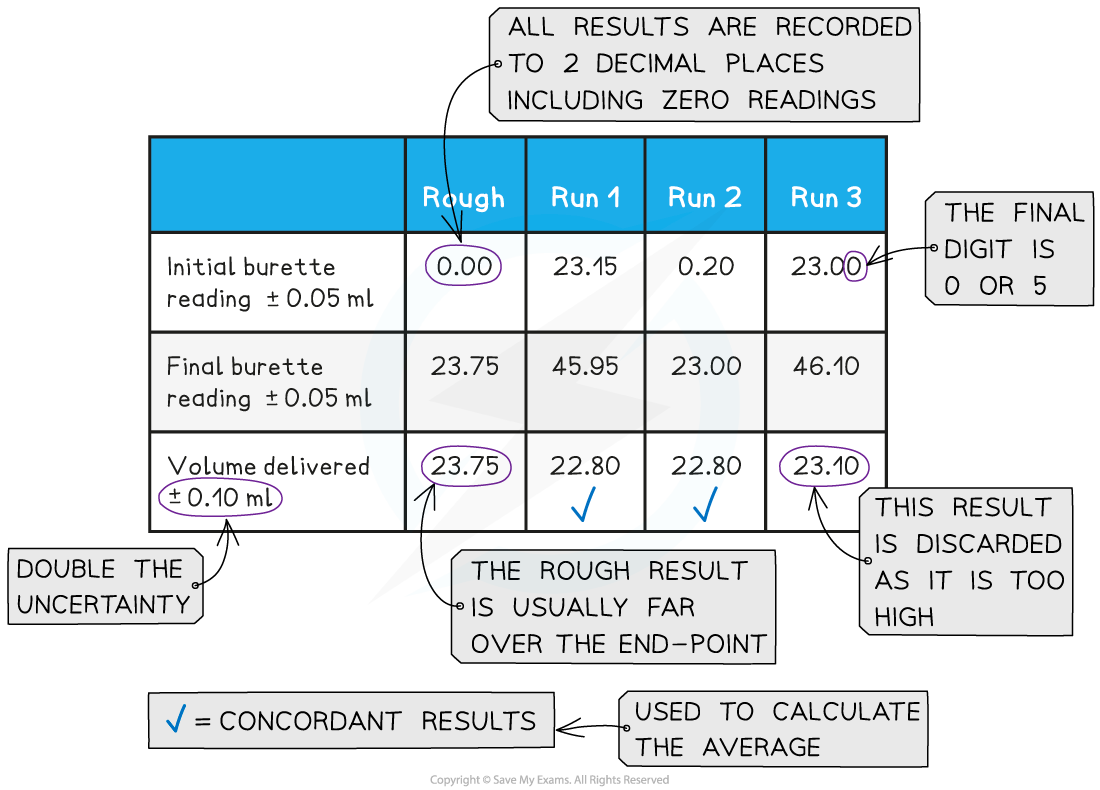
Titration Calculations Aqa A Level . A titration is an experiment that can be used to find the unknown concentration of a solution. Everything you need to know about acids, bases and ph: Use the information to determine the concentration of the hydrochloric acid. Using moles and reacting ratios, you can calculate the concentration of a solution. An uncertainty is the estimated error when taking a measurement. The unknown goes in the conical flask and the known goes in the. Titrations are carried out to. Titration calculations for the a level chemistry aqa exam, totally free, with. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant.
from www.hanlin.com
Titration calculations for the a level chemistry aqa exam, totally free, with. Use the information to determine the concentration of the hydrochloric acid. Everything you need to know about acids, bases and ph: Using moles and reacting ratios, you can calculate the concentration of a solution. A titration is an experiment that can be used to find the unknown concentration of a solution. An uncertainty is the estimated error when taking a measurement. The unknown goes in the conical flask and the known goes in the. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titrations are carried out to.
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育
Titration Calculations Aqa A Level Titrations are carried out to. Titration calculations for the a level chemistry aqa exam, totally free, with. An uncertainty is the estimated error when taking a measurement. Using moles and reacting ratios, you can calculate the concentration of a solution. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. A titration is an experiment that can be used to find the unknown concentration of a solution. Titrations are carried out to. The unknown goes in the conical flask and the known goes in the. Everything you need to know about acids, bases and ph: Use the information to determine the concentration of the hydrochloric acid.
From www.youtube.com
Acids and Bases Back Titration Calculation Exam Question|A Level Titration Calculations Aqa A Level Everything you need to know about acids, bases and ph: The unknown goes in the conical flask and the known goes in the. Titrations are carried out to. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titration calculations for the a level chemistry aqa exam, totally. Titration Calculations Aqa A Level.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Titration Calculations Aqa A Level Titrations are carried out to. An uncertainty is the estimated error when taking a measurement. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titration calculations for the a level chemistry aqa exam, totally free, with. Use the information to determine the concentration of the hydrochloric acid.. Titration Calculations Aqa A Level.
From www.tes.com
AQA Quantitative Chemistry Titration Calculations Teaching Resources Titration Calculations Aqa A Level Use the information to determine the concentration of the hydrochloric acid. Using moles and reacting ratios, you can calculate the concentration of a solution. A titration is an experiment that can be used to find the unknown concentration of a solution. Titrations are carried out to. Everything you need to know about acids, bases and ph: The unknown goes in. Titration Calculations Aqa A Level.
From exozgtvgu.blob.core.windows.net
Titration Gcse Method at Lashandra Cooper blog Titration Calculations Aqa A Level Titration calculations for the a level chemistry aqa exam, totally free, with. Titrations are carried out to. Use the information to determine the concentration of the hydrochloric acid. An uncertainty is the estimated error when taking a measurement. A titration is an experiment that can be used to find the unknown concentration of a solution. A ph curve is a. Titration Calculations Aqa A Level.
From www.revisely.co.uk
ALevel AQA Chemistry Questions pH Curves, Titrations and Indicators Titration Calculations Aqa A Level Titrations are carried out to. Everything you need to know about acids, bases and ph: A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. An uncertainty is the estimated error when taking a measurement. Titration calculations for the a level chemistry aqa exam, totally free, with. Use. Titration Calculations Aqa A Level.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Titration Calculations Aqa A Level Use the information to determine the concentration of the hydrochloric acid. The unknown goes in the conical flask and the known goes in the. Using moles and reacting ratios, you can calculate the concentration of a solution. An uncertainty is the estimated error when taking a measurement. A titration is an experiment that can be used to find the unknown. Titration Calculations Aqa A Level.
From www.tes.com
New AQA GCSE trilogy/chemistrytitration calculations and yield Titration Calculations Aqa A Level An uncertainty is the estimated error when taking a measurement. A titration is an experiment that can be used to find the unknown concentration of a solution. Titrations are carried out to. Everything you need to know about acids, bases and ph: Use the information to determine the concentration of the hydrochloric acid. The unknown goes in the conical flask. Titration Calculations Aqa A Level.
From www.tes.com
Titration for AQA Alevel Chemistry (Slides + Worksheet + Marksheet Titration Calculations Aqa A Level Use the information to determine the concentration of the hydrochloric acid. A titration is an experiment that can be used to find the unknown concentration of a solution. Titrations are carried out to. Everything you need to know about acids, bases and ph: A ph curve is a graphical representation that plots the ph of the solution being titrated against. Titration Calculations Aqa A Level.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Calculations Aqa A Level Use the information to determine the concentration of the hydrochloric acid. Using moles and reacting ratios, you can calculate the concentration of a solution. A titration is an experiment that can be used to find the unknown concentration of a solution. Everything you need to know about acids, bases and ph: A ph curve is a graphical representation that plots. Titration Calculations Aqa A Level.
From fyoczrqcn.blob.core.windows.net
How To Do The Calculations For A Titration at David McClendon blog Titration Calculations Aqa A Level Using moles and reacting ratios, you can calculate the concentration of a solution. Titration calculations for the a level chemistry aqa exam, totally free, with. Titrations are carried out to. The unknown goes in the conical flask and the known goes in the. Everything you need to know about acids, bases and ph: A titration is an experiment that can. Titration Calculations Aqa A Level.
From exosqdnkw.blob.core.windows.net
Titration Calculations A Level Aqa at John Walling blog Titration Calculations Aqa A Level Titrations are carried out to. Using moles and reacting ratios, you can calculate the concentration of a solution. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. The unknown goes in the conical flask and the known goes in the. Everything you need to know about acids,. Titration Calculations Aqa A Level.
From www.savemyexams.com
Titrations (5.6.3) AQA A Level Chemistry Revision Notes 2017 Save Titration Calculations Aqa A Level A titration is an experiment that can be used to find the unknown concentration of a solution. The unknown goes in the conical flask and the known goes in the. Titrations are carried out to. An uncertainty is the estimated error when taking a measurement. Titration calculations for the a level chemistry aqa exam, totally free, with. Using moles and. Titration Calculations Aqa A Level.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Calculations Aqa A Level A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Using moles and reacting ratios, you can calculate the concentration of a solution. Everything you need to know about acids, bases and ph: The unknown goes in the conical flask and the known goes in the. Use the. Titration Calculations Aqa A Level.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Aqa A Level Titrations are carried out to. A titration is an experiment that can be used to find the unknown concentration of a solution. An uncertainty is the estimated error when taking a measurement. Using moles and reacting ratios, you can calculate the concentration of a solution. Titration calculations for the a level chemistry aqa exam, totally free, with. Use the information. Titration Calculations Aqa A Level.
From www.youtube.com
Titration calculations A level chemistry AQA YouTube Titration Calculations Aqa A Level An uncertainty is the estimated error when taking a measurement. The unknown goes in the conical flask and the known goes in the. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Everything you need to know about acids, bases and ph: Using moles and reacting ratios,. Titration Calculations Aqa A Level.
From www.studocu.com
Redox Titrations part 1 AQA Redox Titrations part 1 Fe2+/MnO4 This Titration Calculations Aqa A Level Use the information to determine the concentration of the hydrochloric acid. Everything you need to know about acids, bases and ph: Titrations are carried out to. The unknown goes in the conical flask and the known goes in the. Titration calculations for the a level chemistry aqa exam, totally free, with. A ph curve is a graphical representation that plots. Titration Calculations Aqa A Level.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Titration Calculations Aqa A Level A titration is an experiment that can be used to find the unknown concentration of a solution. Use the information to determine the concentration of the hydrochloric acid. Using moles and reacting ratios, you can calculate the concentration of a solution. An uncertainty is the estimated error when taking a measurement. Titration calculations for the a level chemistry aqa exam,. Titration Calculations Aqa A Level.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Calculations Aqa A Level A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Using moles and reacting ratios, you can calculate the concentration of a solution. Titrations are carried out to. Everything you need to know about acids, bases and ph: Titration calculations for the a level chemistry aqa exam, totally. Titration Calculations Aqa A Level.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Calculations Aqa A Level An uncertainty is the estimated error when taking a measurement. Using moles and reacting ratios, you can calculate the concentration of a solution. Use the information to determine the concentration of the hydrochloric acid. The unknown goes in the conical flask and the known goes in the. Titrations are carried out to. Everything you need to know about acids, bases. Titration Calculations Aqa A Level.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Calculations Aqa A Level A titration is an experiment that can be used to find the unknown concentration of a solution. Using moles and reacting ratios, you can calculate the concentration of a solution. Titrations are carried out to. An uncertainty is the estimated error when taking a measurement. Use the information to determine the concentration of the hydrochloric acid. The unknown goes in. Titration Calculations Aqa A Level.
From www.youtube.com
TITRATION EXAM QUESTION for A LEVEL CHEMISTRY YouTube Titration Calculations Aqa A Level A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. The unknown goes in the conical flask and the known goes in the. Everything you need to know about acids, bases and ph: A titration is an experiment that can be used to find the unknown concentration of. Titration Calculations Aqa A Level.
From baharlabware.com
تیتراسیون و انواع آن تیتراسیون چیست محلول تیتراسیون بهار تجهیز Titration Calculations Aqa A Level Use the information to determine the concentration of the hydrochloric acid. The unknown goes in the conical flask and the known goes in the. An uncertainty is the estimated error when taking a measurement. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titration calculations for the. Titration Calculations Aqa A Level.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育 Titration Calculations Aqa A Level A titration is an experiment that can be used to find the unknown concentration of a solution. Titration calculations for the a level chemistry aqa exam, totally free, with. Titrations are carried out to. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Use the information to. Titration Calculations Aqa A Level.
From www.youtube.com
Titration Calculations YouTube Titration Calculations Aqa A Level Titration calculations for the a level chemistry aqa exam, totally free, with. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Use the information to determine the concentration of the hydrochloric acid. An uncertainty is the estimated error when taking a measurement. Titrations are carried out to.. Titration Calculations Aqa A Level.
From learnah.org
2 PAG2 Carrying out titrations Titration Calculations Aqa A Level Using moles and reacting ratios, you can calculate the concentration of a solution. Everything you need to know about acids, bases and ph: The unknown goes in the conical flask and the known goes in the. A titration is an experiment that can be used to find the unknown concentration of a solution. A ph curve is a graphical representation. Titration Calculations Aqa A Level.
From www.youtube.com
Redox Titrations Exam Questions Alevel Chemistry OCR, AQA, Edexcel Titration Calculations Aqa A Level An uncertainty is the estimated error when taking a measurement. Titrations are carried out to. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. A titration is an experiment that can be used to find the unknown concentration of a solution. Everything you need to know about. Titration Calculations Aqa A Level.
From learnah.org
5 Titration curves and Indicators Titration Calculations Aqa A Level Titration calculations for the a level chemistry aqa exam, totally free, with. Using moles and reacting ratios, you can calculate the concentration of a solution. A titration is an experiment that can be used to find the unknown concentration of a solution. Everything you need to know about acids, bases and ph: Use the information to determine the concentration of. Titration Calculations Aqa A Level.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Calculations Aqa A Level The unknown goes in the conical flask and the known goes in the. Use the information to determine the concentration of the hydrochloric acid. An uncertainty is the estimated error when taking a measurement. A titration is an experiment that can be used to find the unknown concentration of a solution. Titrations are carried out to. Using moles and reacting. Titration Calculations Aqa A Level.
From www.youtube.com
solution to titration of phosphoric acid unit 5 aqa chemistry A2 level Titration Calculations Aqa A Level The unknown goes in the conical flask and the known goes in the. Titration calculations for the a level chemistry aqa exam, totally free, with. A titration is an experiment that can be used to find the unknown concentration of a solution. Everything you need to know about acids, bases and ph: Titrations are carried out to. An uncertainty is. Titration Calculations Aqa A Level.
From exosqdnkw.blob.core.windows.net
Titration Calculations A Level Aqa at John Walling blog Titration Calculations Aqa A Level The unknown goes in the conical flask and the known goes in the. Everything you need to know about acids, bases and ph: Using moles and reacting ratios, you can calculate the concentration of a solution. An uncertainty is the estimated error when taking a measurement. A ph curve is a graphical representation that plots the ph of the solution. Titration Calculations Aqa A Level.
From exosqdnkw.blob.core.windows.net
Titration Calculations A Level Aqa at John Walling blog Titration Calculations Aqa A Level The unknown goes in the conical flask and the known goes in the. Using moles and reacting ratios, you can calculate the concentration of a solution. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. A titration is an experiment that can be used to find the. Titration Calculations Aqa A Level.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Calculations Aqa A Level Using moles and reacting ratios, you can calculate the concentration of a solution. Use the information to determine the concentration of the hydrochloric acid. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. An uncertainty is the estimated error when taking a measurement. Titration calculations for the. Titration Calculations Aqa A Level.
From www.youtube.com
Back Titration Calculations, Paper 1+2 AQA A Level Chemistry YouTube Titration Calculations Aqa A Level An uncertainty is the estimated error when taking a measurement. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Everything you need to know about acids, bases and ph: A titration is an experiment that can be used to find the unknown concentration of a solution. Use. Titration Calculations Aqa A Level.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Calculations Aqa A Level An uncertainty is the estimated error when taking a measurement. Titrations are carried out to. Use the information to determine the concentration of the hydrochloric acid. A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. A titration is an experiment that can be used to find the. Titration Calculations Aqa A Level.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Calculations Aqa A Level A ph curve is a graphical representation that plots the ph of the solution being titrated against the volume of the titrant. Titrations are carried out to. Titration calculations for the a level chemistry aqa exam, totally free, with. A titration is an experiment that can be used to find the unknown concentration of a solution. Using moles and reacting. Titration Calculations Aqa A Level.