Liquid Zirconium . Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. At about 800 °c (1,500 °f) it combines chemically with oxygen to. Zirconium is a chemical element with the symbol zr and atomic number 40. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. This comprehensive guide explores its history,. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2.
from www.semanticscholar.org
At about 800 °c (1,500 °f) it combines chemically with oxygen to. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. This comprehensive guide explores its history,. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. Discover the intriguing world of zirconium, a transitional metal with exceptional properties.
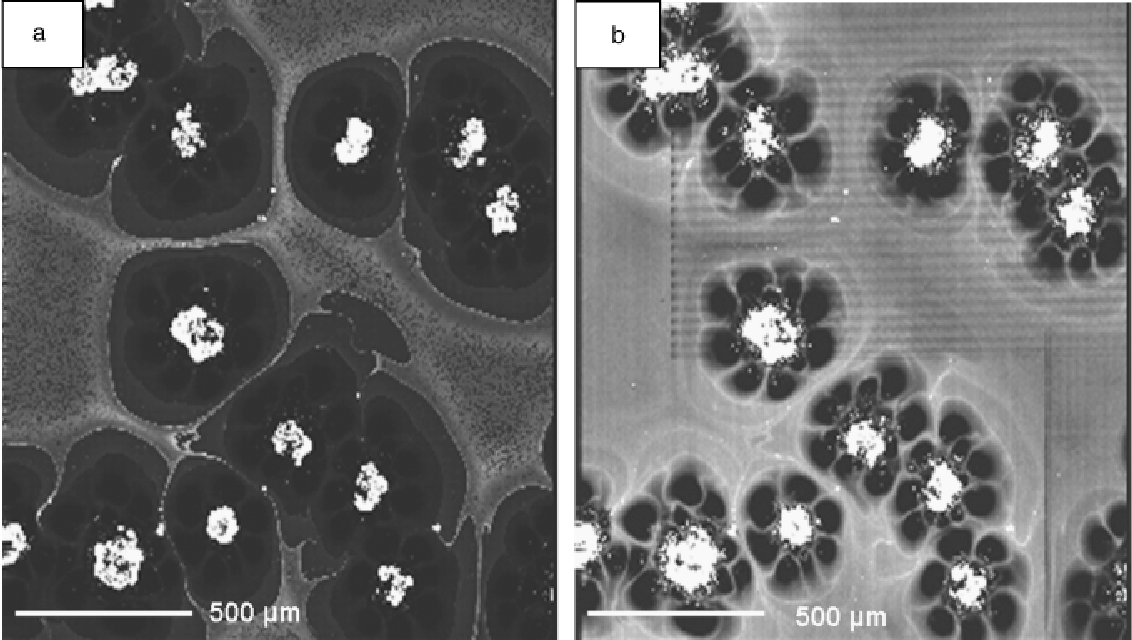
Figure 1 from Zirconia Transport by Liquid Convection during Oxidation
Liquid Zirconium Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. This comprehensive guide explores its history,. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. At about 800 °c (1,500 °f) it combines chemically with oxygen to.
From www.idscad.com
Zirconia Coloring Liquids IDS CAD Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh. Liquid Zirconium.
From www.indiamart.com
Liquid Zirconium Octoate at Rs 15000 Zirconium Octoate in Palghar Liquid Zirconium At about 800 °c (1,500 °f) it combines chemically with oxygen to. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium is in group 4 of the periodic table and is. Liquid Zirconium.
From www.indiamart.com
Zirconium Oxychloride Powder at Rs 600/kg Zirconium Oxychloride in Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium is a chemical element. Liquid Zirconium.
From www.white-peaks-dental.com
Copran Color Zirconium Coloring Liquid 100ml Whitepeaks Dental Solutions Liquid Zirconium Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium is a chemical element with the symbol zr and atomic number 40. This comprehensive guide explores its history,. At about 800 °c (1,500 °f) it combines chemically with oxygen to. Discover the intriguing world of zirconium, a transitional metal with exceptional. Liquid Zirconium.
From www.white-peaks-dental.com
Copran® Color Whitepeaks Dental Solutions Liquid Zirconium Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. This comprehensive guide explores its history,. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Discover the intriguing world of. Liquid Zirconium.
From www.semanticscholar.org
Figure 1 from Zirconia Transport by Liquid Convection during Oxidation Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as. Liquid Zirconium.
From www.youtube.com
How to dye zirconia crown. YouTube Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. This comprehensive guide explores its history,. Zirconium is in group 4 of the periodic table and is. Liquid Zirconium.
From www.indiamart.com
N/A Liquid Zirconium Octoate, 35, Triveni Chemicals ID 1727176688 Liquid Zirconium Zirconium is a chemical element with the symbol zr and atomic number 40. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. This comprehensive guide explores its history,. At. Liquid Zirconium.
From www.dental-zirconiablock.com
Super Translucent 30ml 16 VITA Zirconia Color Liquid FDA Liquid Zirconium Discover the intriguing world of zirconium, a transitional metal with exceptional properties. This comprehensive guide explores its history,. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. At about 800 °c (1,500 °f) it combines chemically with oxygen. Liquid Zirconium.
From www.tradeindia.com
Zirconium Powder, Zirconium Powder Manufacturers & Suppliers, Dealers Liquid Zirconium Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. This comprehensive guide explores its history,. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Zirconium is. Liquid Zirconium.
From www.researchgate.net
(PDF) Crystal nucleation in undercooled liquid zirconium Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. This comprehensive guide explores its history,. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Discover the. Liquid Zirconium.
From www.dentalzirconiablank.com
CE Zirconia Coloring Liquid For Zirconia Teeth Crowns Pink Color Series Liquid Zirconium Zirconium is a chemical element with the symbol zr and atomic number 40. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. At about 800 °c (1,500 °f) it combines chemically with oxygen to. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Zirconium does. Liquid Zirconium.
From www.refractorymetal.org
6 Uses of Zirconium You Mightn't Know Refractory Metals and Alloys Liquid Zirconium Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. This comprehensive guide explores its history,. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. At about 800 °c (1,500 °f) it combines chemically with oxygen. Liquid Zirconium.
From www.researchgate.net
Specific heat capacity of liquid zirconium near the melting point Liquid Zirconium This comprehensive guide explores its history,. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium is a chemical element with the symbol zr and atomic number 40. Discover the intriguing world of zirconium, a transitional metal with exceptional. Liquid Zirconium.
From www.indiamart.com
Liquid Ammonium Zirconium Carbonate, Capacity Plastic Container at Liquid Zirconium This comprehensive guide explores its history,. Zirconium is a chemical element with the symbol zr and atomic number 40. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4,. Liquid Zirconium.
From www.medicalexpo.com
歯冠用歯科材料 Colour Liquid Prettau® Aquarell Opaque Zirkonzahn 液体 / 不透明 Liquid Zirconium Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. Named for the mineral zircon in which. Liquid Zirconium.
From www.academia.edu
(PDF) In situ microscopy observation of liquid flow, zirconia growth Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as. Liquid Zirconium.
From www.dental-zirconiablock.com
One Liter Zirconia Coloring Liquid Ceramic Presintered Zirconia Teeth Liquid Zirconium Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. This comprehensive guide explores its history,. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium oxide. Liquid Zirconium.
From periodictable.com
25ml zirconium crucible, a sample of the element Zirconium in the Liquid Zirconium Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated. Liquid Zirconium.
From www.researchgate.net
Temperature and resistivity (referred to initial dimensions) of Liquid Zirconium Discover the intriguing world of zirconium, a transitional metal with exceptional properties. This comprehensive guide explores its history,. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4,. Liquid Zirconium.
From www.alibaba.com
Dental Stain Liquid Zirconia Dental Liquid Staining Buy Zirconia Liquid Zirconium At about 800 °c (1,500 °f) it combines chemically with oxygen to. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium oxide is used to. Liquid Zirconium.
From dreamstime.com
Zirconium Form Periodic Table Of Elements Royalty Free Stock Photos Liquid Zirconium At about 800 °c (1,500 °f) it combines chemically with oxygen to. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states. Liquid Zirconium.
From www.dreamstime.com
Zirconium Zr Chemical Element Periodic Table Stock Illustration Liquid Zirconium This comprehensive guide explores its history,. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium. Liquid Zirconium.
From www.researchgate.net
Density of liquid zirconium versus temperature. Download Scientific Liquid Zirconium Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Named for the mineral zircon in which it. Liquid Zirconium.
From www.tradeindia.com
Liquid Zirconium Octoate Application Industrial at Best Price in Liquid Zirconium Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. At about 800 °c (1,500 °f) it combines chemically with oxygen to. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium is in group 4 of the periodic. Liquid Zirconium.
From www.idscad.com
Effect Colors IDS CAD Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. At about 800 °c (1,500 °f) it combines chemically with oxygen to. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium. Liquid Zirconium.
From haihangchem.com
zirconium (iv) butoxide cas 1071767 Haihang Industry Liquid Zirconium Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Named for. Liquid Zirconium.
From www.dentalzirconiablank.com
Dental lab liquid for zirconia blocks Liquid Zirconium Zirconium is a chemical element with the symbol zr and atomic number 40. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. Zirconium does not easily react with air. Liquid Zirconium.
From www.gaoyangchem.com
Yixing Gaoyang Chemical Co., Ltd.Zirconium propionateZirconium Liquid Zirconium Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. This comprehensive guide explores its history,. Zirconium absorbs. Liquid Zirconium.
From www.researchgate.net
Specific heat capacity of liquid zirconium near the melting point Liquid Zirconium At about 800 °c (1,500 °f) it combines chemically with oxygen to. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium is a chemical element with the symbol zr and atomic number 40. This comprehensive guide explores its history,. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Zirconium does not easily react. Liquid Zirconium.
From www.britannica.com
Zirconium Chemical Element, Uses, & Properties Britannica Liquid Zirconium Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states such as zrcl 2. This comprehensive. Liquid Zirconium.
From www.researchgate.net
(PDF) HighTemperature Oxygen Dissolution in Liquid Zirconium Liquid Zirconium Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium is a chemical element with the symbol zr and atomic number 40. At about 800 °c (1,500 °f) it combines chemically with oxygen to. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium. Liquid Zirconium.
From www.youtube.com
LUXEN ZIRCONIA Color Liquid Luxen Multi Premium MA2 YouTube Liquid Zirconium Zirconium absorbs oxygen, nitrogen, and hydrogen in astonishing amounts. Named for the mineral zircon in which it can be found, zirconium was discovered in 1789 by klaproth and eventually isolated in. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. Zirconium is a chemical element with the symbol zr and atomic number 40. At about 800 °c. Liquid Zirconium.
From www.dental-zirconiablock.com
One Liter Zirconia Coloring Liquid Ceramic Presintered Zirconia Teeth Liquid Zirconium At about 800 °c (1,500 °f) it combines chemically with oxygen to. Discover the intriguing world of zirconium, a transitional metal with exceptional properties. Zirconium is in group 4 of the periodic table and is a metal that prefers oxidation state (iv), as in the oxide, zro 2 and the chloride zrcl 4, but it can exhibit lower oxidation states. Liquid Zirconium.
From www.dental-zirconiablock.com
16 Shades System Zirconia Coloring Liquid Good Dyeing Effect Crown Liquid Zirconium Discover the intriguing world of zirconium, a transitional metal with exceptional properties. This comprehensive guide explores its history,. Zirconium does not easily react with air or water, making it exceptionally stable and useful for harsh environments. Zirconium oxide is used to make heat resistant crucibles, ceramics and abrasives. At about 800 °c (1,500 °f) it combines chemically with oxygen to.. Liquid Zirconium.