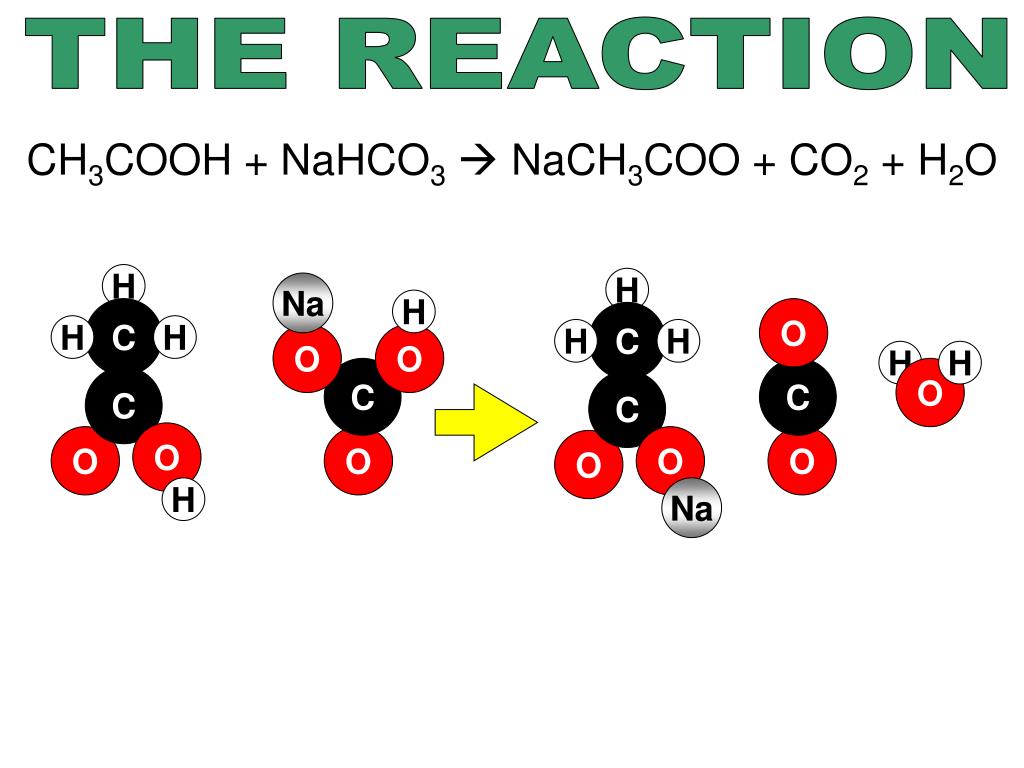
Explain Xkcd Baking Soda And Vinegar . The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The water in the vinegar acts as a host where the base and acid react. The reaction causes the baking soda to transform into water and carbon dioxide. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. This means you're free to copy and share these comics (but not to sell them).
from www.slideserve.com
The water in the vinegar acts as a host where the base and acid react. The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The reaction causes the baking soda to transform into water and carbon dioxide. This means you're free to copy and share these comics (but not to sell them). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid).
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint
Explain Xkcd Baking Soda And Vinegar This means you're free to copy and share these comics (but not to sell them). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. This means you're free to copy and share these comics (but not to sell them). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The reaction causes the baking soda to transform into water and carbon dioxide. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The water in the vinegar acts as a host where the base and acid react.
From www.studypool.com
SOLUTION Vinegar baking soda experiment Studypool Explain Xkcd Baking Soda And Vinegar The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The water in the vinegar acts as a host where the base and acid react. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one. Explain Xkcd Baking Soda And Vinegar.
From handsonaswegrow.com
Baking Soda and Vinegar Experiment to Find Out What's Best! Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The water in the vinegar acts as a host where the base and acid react. The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The overall chemical reaction between baking soda (sodium bicarbonate). Explain Xkcd Baking Soda And Vinegar.
From www.youtube.com
Baking Soda and Vinegar COOL SCIENCE! YouTube Explain Xkcd Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The reaction causes the baking soda to transform into water and carbon dioxide. This means. Explain Xkcd Baking Soda And Vinegar.
From stemmayhem.com
Why Do Vinegar & Baking Soda React? · STEM Mayhem Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. This means you're free to copy and share these comics (but not to sell them). The reaction causes the baking soda to transform into. Explain Xkcd Baking Soda And Vinegar.
From exotjzliw.blob.core.windows.net
Vinegar + Baking Soda Chemical Equation at Allen Oneal blog Explain Xkcd Baking Soda And Vinegar The water in the vinegar acts as a host where the base and acid react. The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid. Explain Xkcd Baking Soda And Vinegar.
From www.apartmenttherapy.com
Don't Mix Baking Soda and Vinegar for Cleaning Apartment Therapy Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The water in the vinegar acts as a host where the base and acid react. This means you're free to copy and share these comics (but not to sell them). The overall chemical reaction between baking soda. Explain Xkcd Baking Soda And Vinegar.
From exoztubcl.blob.core.windows.net
Baking Soda And Vinegar Experiment Formula at Jessica Cuevas blog Explain Xkcd Baking Soda And Vinegar The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each. Explain Xkcd Baking Soda And Vinegar.
From dxodiacqu.blob.core.windows.net
What Can Baking Soda And Vinegar Do at Carl Kron blog Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one. Explain Xkcd Baking Soda And Vinegar.
From www.youtube.com
Baking soda and vinegar reactionscience experiment Harsh Sirohi Explain Xkcd Baking Soda And Vinegar This means you're free to copy and share these comics (but not to sell them). The water in the vinegar acts as a host where the base and acid react. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The amount of vinegar is constant, varying. Explain Xkcd Baking Soda And Vinegar.
From www.mashed.com
Here's What Really Happens When You Mix Vinegar And Baking Soda Explain Xkcd Baking Soda And Vinegar This means you're free to copy and share these comics (but not to sell them). During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The water in the vinegar acts as a host where the base and acid react. The overall chemical reaction between baking soda. Explain Xkcd Baking Soda And Vinegar.
From bazamelanienash.blogspot.com
Baking Soda and Vinegar Reaction Melanie Nash Explain Xkcd Baking Soda And Vinegar The water in the vinegar acts as a host where the base and acid react. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate). Explain Xkcd Baking Soda And Vinegar.
From exozgzoxb.blob.core.windows.net
Vinegar And Baking Soda Lesson Plan at James Ralston blog Explain Xkcd Baking Soda And Vinegar The reaction causes the baking soda to transform into water and carbon dioxide. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one. Explain Xkcd Baking Soda And Vinegar.
From www.youtube.com
The BIGGEST Baking Soda and Vinegar EXPLOSION Easy Science for Kids Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). This means you're free to copy and share these comics (but not to sell them). The water in the vinegar acts as a host where the base and acid react. The overall chemical reaction between baking soda. Explain Xkcd Baking Soda And Vinegar.
From www.youtube.com
baking soda and vinegar experiment baking soda and vinegar reaction Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide. Explain Xkcd Baking Soda And Vinegar.
From xkcd.com
xkcd Baking Soda and Vinegar Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide. Explain Xkcd Baking Soda And Vinegar.
From quizzdbmarutaf0c.z13.web.core.windows.net
Baking Soda And Vinegar Science Fair Project Explain Xkcd Baking Soda And Vinegar The water in the vinegar acts as a host where the base and acid react. This means you're free to copy and share these comics (but not to sell them). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole. Explain Xkcd Baking Soda And Vinegar.
From www.youtube.com
Baking soda and vinegar reaction. ⚗️ 3 Experiments to do with kids ⚗️ Explain Xkcd Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. During the. Explain Xkcd Baking Soda And Vinegar.
From activity-mom.com
Fun Baking Soda and Vinegar Science Experiments The Activity Mom Explain Xkcd Baking Soda And Vinegar This means you're free to copy and share these comics (but not to sell them). During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts. Explain Xkcd Baking Soda And Vinegar.
From klaaevhye.blob.core.windows.net
How Do You Mix Vinegar And Baking Soda at Katherine Nolan blog Explain Xkcd Baking Soda And Vinegar The reaction causes the baking soda to transform into water and carbon dioxide. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one. Explain Xkcd Baking Soda And Vinegar.
From stemtropolis.com
Vinegar and Baking Soda Reaction Experiments STEMtropolis Explain Xkcd Baking Soda And Vinegar The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. During the. Explain Xkcd Baking Soda And Vinegar.
From www.tomsguide.com
Vinegar vs baking soda — which is best at cleaning? Tom's Guide Explain Xkcd Baking Soda And Vinegar The water in the vinegar acts as a host where the base and acid react. This means you're free to copy and share these comics (but not to sell them). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction. Explain Xkcd Baking Soda And Vinegar.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Explain Xkcd Baking Soda And Vinegar The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one. Explain Xkcd Baking Soda And Vinegar.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Explain Xkcd Baking Soda And Vinegar The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The amount. Explain Xkcd Baking Soda And Vinegar.
From activity-mom.com
Fun Baking Soda and Vinegar Science Experiments The Activity Mom Explain Xkcd Baking Soda And Vinegar The water in the vinegar acts as a host where the base and acid react. This means you're free to copy and share these comics (but not to sell them). The reaction causes the baking soda to transform into water and carbon dioxide. The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The overall chemical reaction. Explain Xkcd Baking Soda And Vinegar.
From cenfbmyt.blob.core.windows.net
Baking Soda And Vinegar Experiment Procedure at Kevin Hicks blog Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The water in the vinegar acts as a host where the base and acid react. The reaction causes the baking soda to transform into water and carbon dioxide. The amount of vinegar is constant, varying amounts of. Explain Xkcd Baking Soda And Vinegar.
From www.tomsguide.com
What makes baking soda and vinegar so good at cleaning? Tom's Guide Explain Xkcd Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. During the reaction, when the baking soda is mixed with the vinegar, the baking soda. Explain Xkcd Baking Soda And Vinegar.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium Explain Xkcd Baking Soda And Vinegar This means you're free to copy and share these comics (but not to sell them). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The water in the vinegar acts as a host where the base and acid react. The reaction causes the baking soda to transform into water and carbon dioxide. During the reaction, when. Explain Xkcd Baking Soda And Vinegar.
From www.youtube.com
Vinegar and Baking Soda Part 1 YouTube Explain Xkcd Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. During the reaction, when the baking soda is mixed with the vinegar, the baking soda. Explain Xkcd Baking Soda And Vinegar.
From techrene.com
Cleaning Fridge with Vinegar & Baking Soda Guide Techrene Explain Xkcd Baking Soda And Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. This means you're free to copy and share these comics (but not to sell them).. Explain Xkcd Baking Soda And Vinegar.
From klawqjjkc.blob.core.windows.net
Vinegar And Baking Soda Hypothesis at Geneva Weaver blog Explain Xkcd Baking Soda And Vinegar This means you're free to copy and share these comics (but not to sell them). The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one. Explain Xkcd Baking Soda And Vinegar.
From www.bakingoutsidethebox.com
The Truth About Baking Soda and Vinegar Baking Outside the Box Explain Xkcd Baking Soda And Vinegar During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The reaction causes the baking soda to transform into water and carbon dioxide. The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. The water in the vinegar acts as a host where the. Explain Xkcd Baking Soda And Vinegar.
From www.studocu.com
Baking soda and vinegar ANALYSIS OF BAKING SODA AND VINEGAR Explain Xkcd Baking Soda And Vinegar The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The amount. Explain Xkcd Baking Soda And Vinegar.
From www.numerade.com
SOLVED Baking soda and vinegar react according to the following Explain Xkcd Baking Soda And Vinegar This means you're free to copy and share these comics (but not to sell them). The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one. Explain Xkcd Baking Soda And Vinegar.
From www.youtube.com
Baking Soda and Vinegar Equation and Reaction YouTube Explain Xkcd Baking Soda And Vinegar The amount of vinegar is constant, varying amounts of sodium bicarbonate is independent. During the reaction, when the baking soda is mixed with the vinegar, the baking soda (base) takes a proton from the vinegar (acid). The reaction causes the baking soda to transform into water and carbon dioxide. This means you're free to copy and share these comics (but. Explain Xkcd Baking Soda And Vinegar.
From www.thriftyfun.com
Using Baking Soda and Vinegar on Carpet ThriftyFun Explain Xkcd Baking Soda And Vinegar The reaction causes the baking soda to transform into water and carbon dioxide. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. This means. Explain Xkcd Baking Soda And Vinegar.