Why Do We Use Indicators In The Titration . They are usually weak acids or bases, but their conjugate base or acid. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Indicators are substances whose solutions change color due to changes in ph. These traits are desirable so only a small amount of an indicator is needed. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. If a large amount of indicator is. Both indicators change colour over a specific ph range. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Indicators are substances that change colour when they are added to acidic or alkaline solutions. In many titrations, you use a chemical. When choosing the appropriate indicator, the ph of the equivalence point is very important. A useful indicator has a strong color that changes quickly near its pka. This page assumes that you know.
from cezsemit.blob.core.windows.net
The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very important. In many titrations, you use a chemical. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. A useful indicator has a strong color that changes quickly near its pka. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. They are usually weak acids or bases, but their conjugate base or acid. Indicators are substances that change colour when they are added to acidic or alkaline solutions.
Why Are Different Indicators Used In Titration at Ladonna Anderson blog
Why Do We Use Indicators In The Titration When choosing the appropriate indicator, the ph of the equivalence point is very important. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very important. This page assumes that you know. Both indicators change colour over a specific ph range. In many titrations, you use a chemical. They are usually weak acids or bases, but their conjugate base or acid. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. If a large amount of indicator is. These traits are desirable so only a small amount of an indicator is needed. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Indicators are substances whose solutions change color due to changes in ph. A useful indicator has a strong color that changes quickly near its pka. Indicators are substances that change colour when they are added to acidic or alkaline solutions.
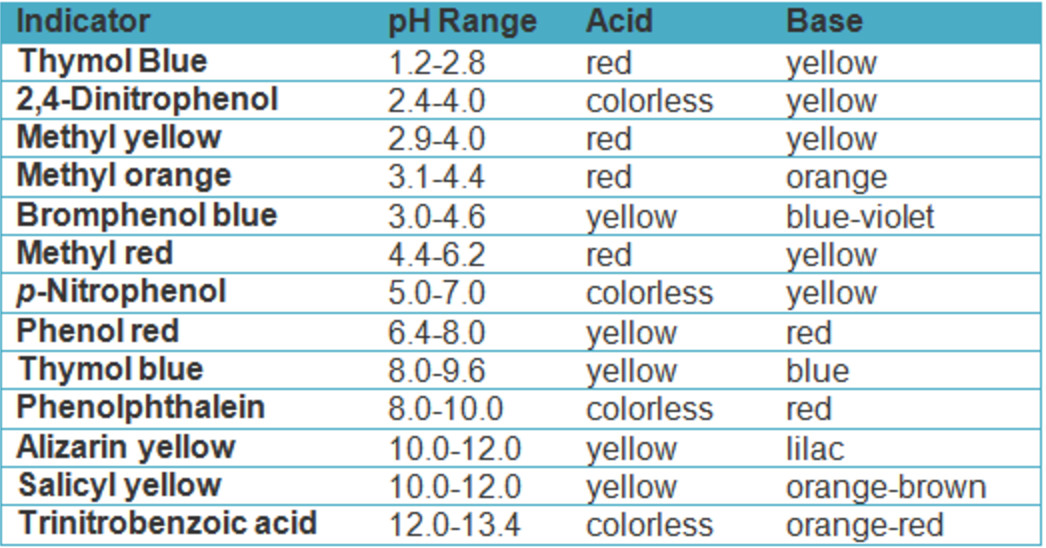
From mungfali.com
Acid Base Titration Indicator Why Do We Use Indicators In The Titration They are usually weak acids or bases, but their conjugate base or acid. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical. If a large amount of indicator. Why Do We Use Indicators In The Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Why Do We Use Indicators In The Titration A useful indicator has a strong color that changes quickly near its pka. Both indicators change colour over a specific ph range. If a large amount of indicator is. In many titrations, you use a chemical. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Some commonly used indicators include. Why Do We Use Indicators In The Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Why Do We Use Indicators In The Titration These traits are desirable so only a small amount of an indicator is needed. They are usually weak acids or bases, but their conjugate base or acid. If a large amount of indicator is. When choosing the appropriate indicator, the ph of the equivalence point is very important. Both indicators change colour over a specific ph range. Indicators are substances. Why Do We Use Indicators In The Titration.
From courses.lumenlearning.com
AcidBase Titrations Boundless Chemistry Why Do We Use Indicators In The Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. If a large amount of indicator is. They are usually weak acids or bases, but their conjugate base or acid. When choosing the appropriate indicator, the ph of the equivalence point is very important. In many titrations, you use a chemical. These traits are desirable. Why Do We Use Indicators In The Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Why Do We Use Indicators In The Titration In many titrations, you use a chemical. Both indicators change colour over a specific ph range. If a large amount of indicator is. A useful indicator has a strong color that changes quickly near its pka. When choosing the appropriate indicator, the ph of the equivalence point is very important. The two most common indicators that are used in titrations. Why Do We Use Indicators In The Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Why Do We Use Indicators In The Titration A useful indicator has a strong color that changes quickly near its pka. These traits are desirable so only a small amount of an indicator is needed. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very important. They are usually weak. Why Do We Use Indicators In The Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Why Do We Use Indicators In The Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. If a large amount of indicator is. A useful indicator has a strong color that changes quickly near its pka. They are usually weak acids or bases, but their conjugate base or acid. Both indicators change colour over a specific ph range. In a titration,. Why Do We Use Indicators In The Titration.
From jeparatd.blogspot.com
Why Does The Indicator Change Color In Titration Jeparat Why Do We Use Indicators In The Titration This page assumes that you know. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In many titrations, you use a chemical. When choosing the appropriate indicator, the ph of the equivalence point is very important. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known. Why Do We Use Indicators In The Titration.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Why Do We Use Indicators In The Titration A useful indicator has a strong color that changes quickly near its pka. These traits are desirable so only a small amount of an indicator is needed. This page assumes that you know. Indicators are substances whose solutions change color due to changes in ph. Indicators are substances that change colour when they are added to acidic or alkaline solutions.. Why Do We Use Indicators In The Titration.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Why Do We Use Indicators In The Titration Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. In many titrations, you use a chemical. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol. Why Do We Use Indicators In The Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Why Do We Use Indicators In The Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical. A useful indicator has a strong color that changes quickly near its pka. Both indicators change colour over a. Why Do We Use Indicators In The Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Why Do We Use Indicators In The Titration Both indicators change colour over a specific ph range. These traits are desirable so only a small amount of an indicator is needed. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. A useful indicator has a strong color that changes quickly near its pka. This page assumes that you know. The. Why Do We Use Indicators In The Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Why Do We Use Indicators In The Titration These traits are desirable so only a small amount of an indicator is needed. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances that change colour when they are added to acidic or alkaline solutions. When choosing the appropriate indicator, the ph of the equivalence point is very important. Both indicators. Why Do We Use Indicators In The Titration.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Why Do We Use Indicators In The Titration This page assumes that you know. A useful indicator has a strong color that changes quickly near its pka. If a large amount of indicator is. When choosing the appropriate indicator, the ph of the equivalence point is very important. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. They. Why Do We Use Indicators In The Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Why Do We Use Indicators In The Titration In many titrations, you use a chemical. A useful indicator has a strong color that changes quickly near its pka. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. If a large amount of indicator is. Both indicators change colour over a specific ph range. When choosing the appropriate indicator, the ph of the. Why Do We Use Indicators In The Titration.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Why Do We Use Indicators In The Titration They are usually weak acids or bases, but their conjugate base or acid. Both indicators change colour over a specific ph range. A useful indicator has a strong color that changes quickly near its pka. If a large amount of indicator is. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. In. Why Do We Use Indicators In The Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Why Do We Use Indicators In The Titration Both indicators change colour over a specific ph range. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Indicators are substances whose solutions change color due to changes in ph. A useful indicator has a strong color that changes quickly near its pka. In many titrations, you use a chemical. The two. Why Do We Use Indicators In The Titration.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Why Do We Use Indicators In The Titration When choosing the appropriate indicator, the ph of the equivalence point is very important. These traits are desirable so only a small amount of an indicator is needed. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A useful indicator has a strong color that changes quickly near its pka. Indicators are substances whose. Why Do We Use Indicators In The Titration.
From freesvg.org
Redox Titration Using Indicator Free SVG Why Do We Use Indicators In The Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions. Both indicators change colour over a specific ph range. A useful indicator has a strong color that changes quickly near its pka. Indicators are substances whose solutions change color due to changes in ph. In many titrations, you use a chemical. These traits are desirable. Why Do We Use Indicators In The Titration.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Why Do We Use Indicators In The Titration A useful indicator has a strong color that changes quickly near its pka. Indicators are substances that change colour when they are added to acidic or alkaline solutions. When choosing the appropriate indicator, the ph of the equivalence point is very important. This page assumes that you know. In a titration, you determine an unknown concentration of a sample by. Why Do We Use Indicators In The Titration.
From www.youtube.com
HOW TO DO DOUBLE INDICATOR TITRATION EXPERIMENTS.Chemistry YouTube Why Do We Use Indicators In The Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions. If a large amount of indicator is. In many titrations, you use a chemical. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. A useful indicator has a strong color. Why Do We Use Indicators In The Titration.
From www.numerade.com
SOLVED Using the table of indicators identify which of the given Why Do We Use Indicators In The Titration They are usually weak acids or bases, but their conjugate base or acid. In many titrations, you use a chemical. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. A useful indicator has a strong color that changes quickly near its pka. Indicators are substances whose solutions change color due to changes. Why Do We Use Indicators In The Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Why Do We Use Indicators In The Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. If a large amount of indicator is. A useful indicator has a strong color that changes quickly near its pka. In many titrations, you use a chemical. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. This. Why Do We Use Indicators In The Titration.
From www.studocu.com
Practicum 2 1. Why do we use indicators in acidbase titration? an Why Do We Use Indicators In The Titration Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. This page assumes that you know. A useful indicator has a strong color that changes quickly near its pka. In many titrations, you use a chemical. Indicators are substances that change colour when they are added to acidic or. Why Do We Use Indicators In The Titration.
From mungfali.com
Acid Base Titration Indicator Why Do We Use Indicators In The Titration This page assumes that you know. When choosing the appropriate indicator, the ph of the equivalence point is very important. Indicators are substances that change colour when they are added to acidic or alkaline solutions. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid.. Why Do We Use Indicators In The Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Why Do We Use Indicators In The Titration In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Indicators are substances that change colour when they are added to acidic or alkaline solutions. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. If a large amount of indicator is. When choosing the appropriate. Why Do We Use Indicators In The Titration.
From www.studypool.com
SOLUTION Acid base indicators titration curves Studypool Why Do We Use Indicators In The Titration These traits are desirable so only a small amount of an indicator is needed. A useful indicator has a strong color that changes quickly near its pka. When choosing the appropriate indicator, the ph of the equivalence point is very important. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration.. Why Do We Use Indicators In The Titration.
From bramblechemistry.weebly.com
4C6 Titration Why Do We Use Indicators In The Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. They are usually weak acids or bases, but their conjugate base or acid. These traits are desirable so only a small amount of an indicator is needed. In many titrations, you use a chemical. Both indicators change colour over a specific ph range. If a. Why Do We Use Indicators In The Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Why Do We Use Indicators In The Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions. If a large amount of indicator is. A useful indicator has a strong color that changes quickly near its pka. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. When choosing the appropriate indicator, the ph of. Why Do We Use Indicators In The Titration.
From cezsemit.blob.core.windows.net
Why Are Different Indicators Used In Titration at Ladonna Anderson blog Why Do We Use Indicators In The Titration In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. They are usually weak acids or bases, but their conjugate base or acid. In many titrations, you use a chemical. These traits are desirable so only. Why Do We Use Indicators In The Titration.
From www.homeazing.com
Why Do We Use Indicators In The Titration They are usually weak acids or bases, but their conjugate base or acid. If a large amount of indicator is. A useful indicator has a strong color that changes quickly near its pka. This page assumes that you know. When choosing the appropriate indicator, the ph of the equivalence point is very important. These traits are desirable so only a. Why Do We Use Indicators In The Titration.
From exocowbmz.blob.core.windows.net
Indicator Used In Calcium Titration at Hattie Murphy blog Why Do We Use Indicators In The Titration If a large amount of indicator is. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Indicators are substances that change colour when they are added to acidic or alkaline solutions. When choosing the. Why Do We Use Indicators In The Titration.
From mungfali.com
Acid Base Titration Indicator Why Do We Use Indicators In The Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. When choosing the appropriate indicator, the ph of the equivalence point is very important. These traits are desirable so only a small amount of an indicator is needed. If a large amount of indicator is. Both indicators change colour over a specific ph range. Indicators. Why Do We Use Indicators In The Titration.
From cezsemit.blob.core.windows.net
Why Are Different Indicators Used In Titration at Ladonna Anderson blog Why Do We Use Indicators In The Titration A useful indicator has a strong color that changes quickly near its pka. In many titrations, you use a chemical. Indicators are substances whose solutions change color due to changes in ph. When choosing the appropriate indicator, the ph of the equivalence point is very important. This page assumes that you know. In a titration, you determine an unknown concentration. Why Do We Use Indicators In The Titration.
From cezsemit.blob.core.windows.net
Why Are Different Indicators Used In Titration at Ladonna Anderson blog Why Do We Use Indicators In The Titration If a large amount of indicator is. A useful indicator has a strong color that changes quickly near its pka. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Both indicators change colour over a specific ph range. These traits are desirable so only a small amount of an indicator. Why Do We Use Indicators In The Titration.