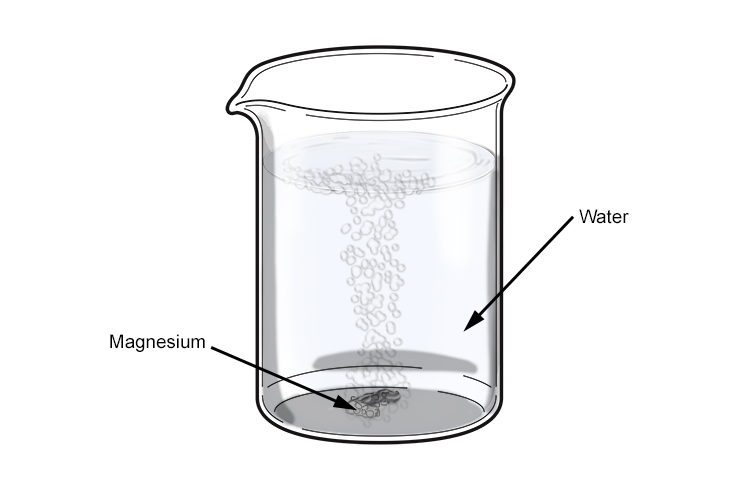
Magnesium And Water Reaction Equation . This video shows the physical properties of mg metal and its reaction with water. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. The reaction between magnesium and water can be represented by the following balanced chemical equation: In this video we'll look at the reaction of mg. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Part of ncssm core collection: Mg (s) + 2h_ {2}o (l) → mg. The reaction of magnesium metal and water comes up quite frequently in chemistry. The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen.
from ar.inspiredpencil.com
Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. This video shows the physical properties of mg metal and its reaction with water. The reaction between magnesium and water can be represented by the following balanced chemical equation: Part of ncssm core collection: The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. In this video we'll look at the reaction of mg.
Magnesium And Water
Magnesium And Water Reaction Equation This video shows the physical properties of mg metal and its reaction with water. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Part of ncssm core collection: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. In this video we'll look at the reaction of mg. The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). The reaction between magnesium and water can be represented by the following balanced chemical equation: Mg (s) + 2h_ {2}o (l) → mg. This video shows the physical properties of mg metal and its reaction with water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. The reaction of magnesium metal and water comes up quite frequently in chemistry.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and Magnesium And Water Reaction Equation The reaction of magnesium metal and water comes up quite frequently in chemistry. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. This video shows the physical properties of mg metal and its reaction with water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium. Magnesium And Water Reaction Equation.
From www.youtube.com
How to Balance Mg + H2O = MgO + H2 Magnesium + Water (steam) YouTube Magnesium And Water Reaction Equation The reaction of magnesium metal and water comes up quite frequently in chemistry. Mg (s) + 2h_ {2}o (l) → mg. The reaction between magnesium and water can be represented by the following balanced chemical equation: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. In this video we'll look at the reaction of mg. The equation. Magnesium And Water Reaction Equation.
From www.scribd.com
Magnesium Reacts With Dilute Hydrochloric Acid in a Conical Flask Which Magnesium And Water Reaction Equation In this video we'll look at the reaction of mg. This video shows the physical properties of mg metal and its reaction with water. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. The reaction between magnesium and water can be represented by the following balanced chemical equation: When exposed to steam,. Magnesium And Water Reaction Equation.
From ar.inspiredpencil.com
Magnesium And Water Magnesium And Water Reaction Equation Mg (s) + 2h_ {2}o (l) → mg. In this video we'll look at the reaction of mg. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and. Magnesium And Water Reaction Equation.
From www.youtube.com
Why does Magnesium react with Water? YouTube Magnesium And Water Reaction Equation In this video we'll look at the reaction of mg. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Mg (s) + 2h_ {2}o (l) → mg. The reaction of magnesium metal and water comes up quite frequently in chemistry. When exposed to steam, magnesium changes from magnesium to magnesium oxide and. Magnesium And Water Reaction Equation.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium And Water Reaction Equation The reaction between magnesium and water can be represented by the following balanced chemical equation: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health.. Magnesium And Water Reaction Equation.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium And Water Reaction Equation Part of ncssm core collection: When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. In this video we'll look at the reaction of mg. Mg (s) + 2h_ {2}o (l) → mg.. Magnesium And Water Reaction Equation.
From www.numerade.com
SOLVED write a balanced chemical equation for the dissociation of Magnesium And Water Reaction Equation When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. The reaction of magnesium metal and water comes up quite frequently in chemistry. The reaction between magnesium and water can be represented by the following balanced chemical equation: The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). In this video. Magnesium And Water Reaction Equation.
From solvedlib.com
Magnesium nitride reacts with water to form ammonia a… SolvedLib Magnesium And Water Reaction Equation When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. The reaction of magnesium metal and water comes up quite frequently in chemistry. Part of ncssm core collection: In this video we'll look at the reaction of mg. Mg (s) + 2h_ {2}o (l) → mg. This video shows the physical properties of. Magnesium And Water Reaction Equation.
From www.numerade.com
SOLVED Write a balanced equation for reaction of the basic oxide Magnesium And Water Reaction Equation In this video we'll look at the reaction of mg. The reaction of magnesium metal and water comes up quite frequently in chemistry. Part of ncssm core collection: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. The reaction between magnesium and water can be represented by the following balanced chemical equation: When exposed to steam, magnesium. Magnesium And Water Reaction Equation.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium And Water Reaction Equation Mg (s) + 2h_ {2}o (l) → mg. This video shows the physical properties of mg metal and its reaction with water. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. The. Magnesium And Water Reaction Equation.
From www.slideshare.net
Reactions & Formulas Magnesium And Water Reaction Equation Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Magnesium burns in steam to produce white magnesium oxide and hydrogen. Magnesium And Water Reaction Equation.
From www.slideserve.com
PPT Variation in Chemical Properties of sblock elements PowerPoint Magnesium And Water Reaction Equation The reaction between magnesium and water can be represented by the following balanced chemical equation: \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Part of ncssm core collection: The reaction of magnesium metal. Magnesium And Water Reaction Equation.
From www.numerade.com
SOLVED Consider the reaction between magnesium nitride and water where Magnesium And Water Reaction Equation The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. The reaction between magnesium and water can be represented by the following balanced chemical equation: Magnesium burns in steam to produce. Magnesium And Water Reaction Equation.
From www.youtube.com
Magnesium reacting with Water. YouTube Magnesium And Water Reaction Equation This video shows the physical properties of mg metal and its reaction with water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. Part of ncssm core collection: The reaction of magnesium metal and water comes up quite frequently in chemistry. The reaction between magnesium and. Magnesium And Water Reaction Equation.
From ar.inspiredpencil.com
Magnesium And Water Magnesium And Water Reaction Equation When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. In this video we'll look at the reaction of mg. The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). The reaction between magnesium and water can be represented by the following balanced chemical equation: Learn how. Magnesium And Water Reaction Equation.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium And Water Reaction Equation When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Mg (s) + 2h_ {2}o (l) → mg. This video shows the physical properties of mg metal and its reaction with water. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Magnesium burns in steam to produce white magnesium oxide. Magnesium And Water Reaction Equation.
From www.slideshare.net
Metals Magnesium And Water Reaction Equation Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. In this video we'll look at the reaction of mg. The reaction between magnesium and water can be represented by the following balanced chemical equation: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When exposed to steam, magnesium changes from magnesium to magnesium oxide. Magnesium And Water Reaction Equation.
From www.youtube.com
Reaction of Magnesium and Water (Mg + H2O) YouTube Magnesium And Water Reaction Equation Part of ncssm core collection: Mg (s) + 2h_ {2}o (l) → mg. The reaction between magnesium and water can be represented by the following balanced chemical equation: This video shows the physical properties of mg metal and its reaction with water. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. When exposed to steam, magnesium changes. Magnesium And Water Reaction Equation.
From www.tessshebaylo.com
Magnesium And Hot Water Equation Tessshebaylo Magnesium And Water Reaction Equation Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. The reaction of magnesium metal and water comes up quite frequently in chemistry. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. This video shows the physical properties of mg metal and its reaction with water. When. Magnesium And Water Reaction Equation.
From www.slideshare.net
Chemical Reactions Magnesium And Water Reaction Equation The reaction of magnesium metal and water comes up quite frequently in chemistry. Part of ncssm core collection: The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. In this video we'll look at the reaction of. Magnesium And Water Reaction Equation.
From www.sciencephoto.com
Magnesium reacting with water Stock Image A500/0369 Science Photo Magnesium And Water Reaction Equation The reaction between magnesium and water can be represented by the following balanced chemical equation: In this video we'll look at the reaction of mg. Part of ncssm core collection: The reaction of magnesium metal and water comes up quite frequently in chemistry. This video shows the physical properties of mg metal and its reaction with water. The equation is. Magnesium And Water Reaction Equation.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O Magnesium And Water Reaction Equation Mg (s) + 2h_ {2}o (l) → mg. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. In this video we'll look at the reaction of mg. Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. When exposed to steam, magnesium changes from magnesium to magnesium. Magnesium And Water Reaction Equation.
From www.nagwa.com
Question Video Balancing a Chemical Equation for the Reaction between Magnesium And Water Reaction Equation The reaction of magnesium metal and water comes up quite frequently in chemistry. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. Mg (s) + 2h_ {2}o (l) → mg. The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g).. Magnesium And Water Reaction Equation.
From www.teachoo.com
Chemical Properties of Metals [with Reaction Examples] Teachoo Magnesium And Water Reaction Equation The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. The reaction of magnesium metal and water. Magnesium And Water Reaction Equation.
From www.sciencephoto.com
Magnesium reacts with water Stock Image C028/0749 Science Photo Magnesium And Water Reaction Equation Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. In this video. Magnesium And Water Reaction Equation.
From www.youtube.com
Experiment 3 Magnesium water reaction YouTube Magnesium And Water Reaction Equation Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. This video shows the physical properties of mg metal and its reaction with water. When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. When magnesium reacts with liquid water, it makes mg. Magnesium And Water Reaction Equation.
From ar.inspiredpencil.com
Magnesium And Water Magnesium And Water Reaction Equation In this video we'll look at the reaction of mg. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Mg (s) + 2h_ {2}o (l) → mg. Part of ncssm core collection: Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. This video shows the physical properties of mg. Magnesium And Water Reaction Equation.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium And Water Reaction Equation The reaction of magnesium metal and water comes up quite frequently in chemistry. Magnesium burns in steam to produce white magnesium oxide and hydrogen gas. In this video we'll look at the reaction of mg. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Learn how magnesium reacts with water vapor, water. Magnesium And Water Reaction Equation.
From www.alamy.com
Labelled diagram of magnesium reacting with steam. Vector diagram for Magnesium And Water Reaction Equation The reaction of magnesium metal and water comes up quite frequently in chemistry. This video shows the physical properties of mg metal and its reaction with water. Part of ncssm core collection: \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon has a mild reaction with cold water, given below. The reaction between magnesium and. Magnesium And Water Reaction Equation.
From www.numerade.com
SOLVEDThe reaction of magnesium with water can be used as a means for Magnesium And Water Reaction Equation When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. Mg (s) + 2h_ {2}o (l) → mg. In this video we'll look at the reaction of mg. Part of ncssm core collection: The equation is mg (s) + h 2 o (g) mgo (s) + h 2 (g). Learn how magnesium reacts with water vapor, water. Magnesium And Water Reaction Equation.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium And Water Reaction Equation The reaction between magnesium and water can be represented by the following balanced chemical equation: Learn how magnesium reacts with water vapor, water and other compounds, and how it affects water hardness, environment and health. When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. The reaction of magnesium metal and water comes. Magnesium And Water Reaction Equation.
From www.youtube.com
Equation for MgI2 + H2O (Magnesium iodide + Water) YouTube Magnesium And Water Reaction Equation When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. The reaction between magnesium and water can be represented by the following balanced chemical equation: In this video we'll look at the reaction of mg. Mg (s) + 2h_ {2}o (l) → mg. Learn how magnesium reacts with water vapor, water and other compounds, and how it. Magnesium And Water Reaction Equation.
From www.researchgate.net
PotentialpH equilibrium diagram for magnesiumwater system at 25 °C Magnesium And Water Reaction Equation When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. Part of ncssm core collection: In this video we'll look at the reaction of mg. This video shows the physical properties of mg metal and its reaction with water. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)} \label{1} \] very clean magnesium ribbon. Magnesium And Water Reaction Equation.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Magnesium And Water Reaction Equation When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. Part of ncssm core collection: When magnesium reacts with liquid water, it makes mg (oh)2 in a regular old single displacement reaction. In this video we'll look at the reaction of mg. This video shows the physical properties of mg metal and its reaction with water. The. Magnesium And Water Reaction Equation.