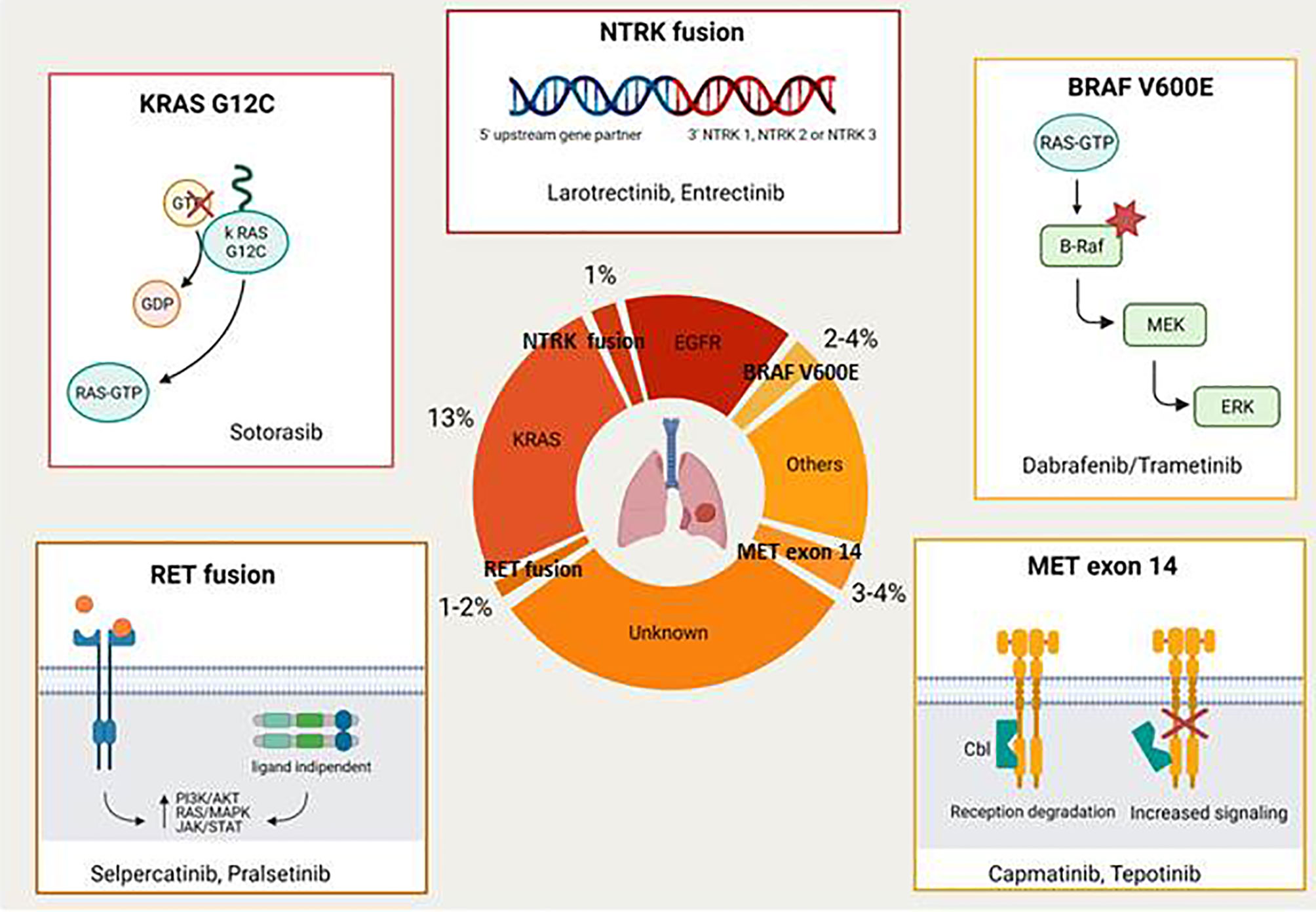
Braf Gene Therapy . the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort.
from www.frontiersin.org
braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the.
Frontiers Beyond Conventional The New Horizon of Targeted Therapy
Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort.
From www.cell.com
Clinical Development of BRAF plus MEK Inhibitor Combinations Trends in Braf Gene Therapy testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations. Braf Gene Therapy.
From www.thelancet.com
Systemic treatment for BRAFmutant melanoma where do we go next? The Braf Gene Therapy with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf inhibitor resistance is common. Braf Gene Therapy.
From www.semanticscholar.org
Figure 1 from Diverse BRAF Gene Fusions Confer Resistance to EGFR Braf Gene Therapy braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. testing for braf mutations enables patients to be. Braf Gene Therapy.
From www.cancer.gov
Targeted Drug Trio for Colorectal Cancer with BRAF Mutations NCI Braf Gene Therapy with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). testing for braf mutations. Braf Gene Therapy.
From debuglies.com
New therapy for advanced melanoma BRAF V600E Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf mutations are frequently found. Braf Gene Therapy.
From www.cell.com
Targeted Disruption of V600EMutant BRAF Gene by CRISPRCpf1 Molecular Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. braf inhibitor resistance is common and. Braf Gene Therapy.
From www.sohu.com
综述 甲状腺癌(Thyroid Cancer)发病相关RNA分子机制研究进展搜狐大视野搜狐新闻 Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf inhibitor resistance is common and. Braf Gene Therapy.
From gut.bmj.com
A combined oncogenic pathway signature of BRAF, KRAS and PI3KCA Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). testing for braf mutations enables patients. Braf Gene Therapy.
From jgo.amegroups.com
BRAF mutant colorectal cancer as a distinct subset of colorectal cancer Braf Gene Therapy testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations. Braf Gene Therapy.
From translational-medicine.biomedcentral.com
The role of BRAF V600 mutation in melanoma Journal of Translational Braf Gene Therapy braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the fda approved amtagvi, the first cellular therapy for the treatment. Braf Gene Therapy.
From www.mdpi.com
Cancers Free FullText Molecular Heterogeneity in BRAFMutant Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. the food and drug administration (fda) recently approved the combination. Braf Gene Therapy.
From www.frontiersin.org
Frontiers Therapeutic strategies for BRAF mutation in nonsmall cell Braf Gene Therapy testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). with the identification of activating. Braf Gene Therapy.
From www.semanticscholar.org
Figure 2 from Diverse BRAF Gene Fusions Confer Resistance to EGFR Braf Gene Therapy with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. testing for braf mutations enables patients to be. Braf Gene Therapy.
From www.frontiersin.org
Frontiers BRAFMutated NonSmall Cell Lung Cancer Current Treatment Braf Gene Therapy braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the food and drug administration (fda) recently approved. Braf Gene Therapy.
From www.personalizedmedonc.com
Personalized Medicine in Oncology BRAF Mutations An Old Oncogene and Braf Gene Therapy braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations are frequently found in. Braf Gene Therapy.
From ascopubs.org
BRAFMutated Advanced Colorectal Cancer A Rapidly Changing Therapeutic Braf Gene Therapy braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations in braf across a. Braf Gene Therapy.
From www.pinterest.com
BRAF Geni Mutasyonu BRAF gene mutation Bronzlaşma Braf Gene Therapy braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. with the identification of activating mutations in braf across. Braf Gene Therapy.
From www.semanticscholar.org
Figure 1 from Diverse BRAF Gene Fusions Confer Resistance to EGFR Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. braf mutations are frequently found in. Braf Gene Therapy.
From ilovepathology.com
BRAF Gene and "BRAFoma's" Pathology Made Simple Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. testing for braf mutations enables patients to. Braf Gene Therapy.
From www.frontiersin.org
Frontiers Management of BRAF Gene Alterations in Metastatic Braf Gene Therapy testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. braf inhibitor resistance is common and manifests. Braf Gene Therapy.
From jnccn.org
BRAF Mutations in Colorectal Cancer Clinical Relevance and Role in Braf Gene Therapy with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). testing for braf mutations enables patients to. Braf Gene Therapy.
From www.mdpi.com
Cancers Free FullText BRAF V600Mutated Metastatic Melanoma and Braf Gene Therapy with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. braf mutations are frequently found in. Braf Gene Therapy.
From mavink.com
Braf Mapk Pathway Braf Gene Therapy testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. braf mutations are. Braf Gene Therapy.
From www.mdpi.com
IJMS Free FullText BRAFMutated Colorectal Cancer Clinical and Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. with the identification of. Braf Gene Therapy.
From www.frontiersin.org
Frontiers Beyond Conventional The New Horizon of Targeted Therapy Braf Gene Therapy with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. testing for braf mutations enables patients to be treated. Braf Gene Therapy.
From www.mdpi.com
Pharmaceutics Free FullText Targeting BRAF Activation as Acquired Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf inhibitor resistance is common. Braf Gene Therapy.
From www.esmo.org
Comprehensive Genomic Profiling of BRAF in a Large Series of Lung Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. with the identification of activating mutations. Braf Gene Therapy.
From www2.mdpi.com
IJMS Free FullText NonSmall Cell Lung Cancer Targeted Therapy Braf Gene Therapy braf inhibitor resistance is common and manifests through rapid reactivation of erk signalling, owing to. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). with the identification of. Braf Gene Therapy.
From www.cell.com
RASopathy mutations open new insights into the mechanism of BRAF Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf mutations are. Braf Gene Therapy.
From www.semanticscholar.org
Figure 5 from Diverse BRAF Gene Fusions Confer Resistance to EGFR Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. testing for braf mutations enables patients to. Braf Gene Therapy.
From www.researchgate.net
BRAF mutation distribution in various cancer types and protein Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. braf inhibitor resistance is. Braf Gene Therapy.
From www.semanticscholar.org
Figure 2 from Diverse BRAF Gene Fusions Confer Resistance to EGFR Braf Gene Therapy the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations. Braf Gene Therapy.
From studylib.net
BRAF and Malignant Melanoma From gene to cancer therapy Braf Gene Therapy testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. braf mutations are frequently found in melanoma and targeted therapies against these specific genetic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the food and drug administration (fda) recently approved. Braf Gene Therapy.
From www.researchgate.net
BRAFmediated signaling in normal and cancer cells. In normal cells Braf Gene Therapy with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. the food and drug administration (fda) recently approved the combination of the targeted drugs dabrafenib (tafinlar) and trametinib (mekinist). braf mutations are frequently. Braf Gene Therapy.
From atlasofscience.org
BRAF inhibitor and interferon alpha combination for melanoma treatment Braf Gene Therapy the fda approved amtagvi, the first cellular therapy for the treatment of adults with unresectable or metastatic. with the identification of activating mutations in braf across a wide variety of malignancies, substantial effort. testing for braf mutations enables patients to be treated with therapies that directly target braf v600e and the. the food and drug administration. Braf Gene Therapy.