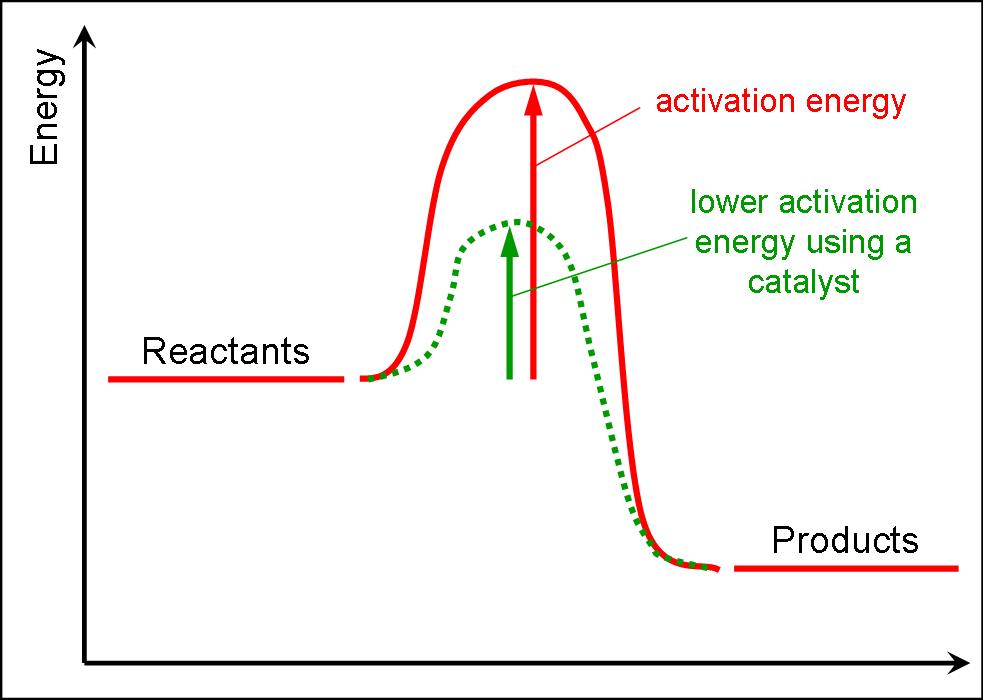
Is The Difference Between Threshold Energy And Activation Energy . In other words, the activation energy is what. The difference between the quantities is briefly outlined in iupac green book [1, pp. The term activation energy is also used for an. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. It is equal to the difference between the. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. Lower activation energy means faster reaction. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. Activation energy plays a crucial role in determining the rate of a chemical reaction; The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy.
from professional.patrickneyman.com
The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. Activation energy plays a crucial role in determining the rate of a chemical reaction; Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. The term activation energy is also used for an. The difference between the quantities is briefly outlined in iupac green book [1, pp. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. Lower activation energy means faster reaction. It is equal to the difference between the.
Activation Energy Equation
Is The Difference Between Threshold Energy And Activation Energy It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. The difference between the quantities is briefly outlined in iupac green book [1, pp. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. It is equal to the difference between the. The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. In other words, the activation energy is what. The term activation energy is also used for an. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. Lower activation energy means faster reaction. Activation energy plays a crucial role in determining the rate of a chemical reaction;
From blogs.glowscotland.org.uk
Activation Energy Higher Chemistry Unit 1 Is The Difference Between Threshold Energy And Activation Energy Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The term activation energy is also used for an. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. Lower activation energy means faster reaction. The difference between the quantities is briefly outlined in. Is The Difference Between Threshold Energy And Activation Energy.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Is The Difference Between Threshold Energy And Activation Energy In other words, the activation energy is what. The term activation energy is also used for an. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The term that refers to. Is The Difference Between Threshold Energy And Activation Energy.
From pediaa.com
Difference Between Energy and Activation Energy Definition, Forms of Is The Difference Between Threshold Energy And Activation Energy The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. Lower activation energy means faster reaction. It is equal to the difference between the. The difference between the quantities is briefly outlined. Is The Difference Between Threshold Energy And Activation Energy.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Is The Difference Between Threshold Energy And Activation Energy The term activation energy is also used for an. In other words, the activation energy is what. The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. Lower activation energy means faster reaction. It is equal to the difference between the. The difference between the quantities is. Is The Difference Between Threshold Energy And Activation Energy.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Is The Difference Between Threshold Energy And Activation Energy Lower activation energy means faster reaction. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in. Is The Difference Between Threshold Energy And Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Is The Difference Between Threshold Energy And Activation Energy Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. In other words, the activation energy is what. The term activation energy is also used for an. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. The term that refers to the difference. Is The Difference Between Threshold Energy And Activation Energy.
From circuitdiagramlows.z22.web.core.windows.net
What Is The Activation Energy On A Diagram Is The Difference Between Threshold Energy And Activation Energy Lower activation energy means faster reaction. Activation energy plays a crucial role in determining the rate of a chemical reaction; It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. In other words, the. Is The Difference Between Threshold Energy And Activation Energy.
From sciencenotes.org
What Is Activation Energy? Definition and Examples Is The Difference Between Threshold Energy And Activation Energy It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. The difference between the quantities is briefly outlined in iupac green book [1, pp. The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. Activation energy plays a. Is The Difference Between Threshold Energy And Activation Energy.
From scienceinfo.com
Activation Energy Definition, Unit, Formula, Calculations Is The Difference Between Threshold Energy And Activation Energy The difference between the quantities is briefly outlined in iupac green book [1, pp. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The excess energy which must be supplied to the reactants. Is The Difference Between Threshold Energy And Activation Energy.
From telegra.ph
Endothermic energy profile diagram activation Telegraph Is The Difference Between Threshold Energy And Activation Energy In other words, the activation energy is what. Activation energy plays a crucial role in determining the rate of a chemical reaction; It is equal to the difference between the. Lower activation energy means faster reaction. The term activation energy is also used for an. Activation energy is primarily associated with chemical reactions, where it represents the energy required for. Is The Difference Between Threshold Energy And Activation Energy.
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of Is The Difference Between Threshold Energy And Activation Energy It is equal to the difference between the. Activation energy plays a crucial role in determining the rate of a chemical reaction; The term activation energy is also used for an. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. The excess energy which must be supplied to the reactants. Is The Difference Between Threshold Energy And Activation Energy.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Is The Difference Between Threshold Energy And Activation Energy It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. The difference between the quantities is briefly outlined in iupac green book [1, pp. Lower activation energy means. Is The Difference Between Threshold Energy And Activation Energy.
From www.expii.com
Energy Diagram — Overview & Parts Expii Is The Difference Between Threshold Energy And Activation Energy Lower activation energy means faster reaction. The difference between the quantities is briefly outlined in iupac green book [1, pp. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. In other words, the activation energy is what. The excess energy which must be supplied to the reactants to undergo chemical reactions is. Is The Difference Between Threshold Energy And Activation Energy.
From www.doubtnut.com
[Odia] What is the difference between activation energy and threshold Is The Difference Between Threshold Energy And Activation Energy In other words, the activation energy is what. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The difference between the quantities is briefly outlined in iupac green book [1, pp. It is equal to the difference between the. The term activation energy is also used for an. The difference. Is The Difference Between Threshold Energy And Activation Energy.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Is The Difference Between Threshold Energy And Activation Energy Lower activation energy means faster reaction. The term activation energy is also used for an. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. Activation energy plays a crucial role in determining the rate of a chemical reaction; It is equal to the difference between the. Activation energy is primarily. Is The Difference Between Threshold Energy And Activation Energy.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Is The Difference Between Threshold Energy And Activation Energy Lower activation energy means faster reaction. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. In other words, the activation energy is what. The term activation energy is also used for an. It is equal to the difference between the. The term that refers to the difference between the energy. Is The Difference Between Threshold Energy And Activation Energy.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID239015 Is The Difference Between Threshold Energy And Activation Energy The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. Activation energy plays a crucial role in determining the rate of a chemical reaction; The difference between the. Is The Difference Between Threshold Energy And Activation Energy.
From socratic.org
What are activation energies? Socratic Is The Difference Between Threshold Energy And Activation Energy Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. It. Is The Difference Between Threshold Energy And Activation Energy.
From www.slideserve.com
PPT CHAPTER 3 PowerPoint Presentation, free download ID1361511 Is The Difference Between Threshold Energy And Activation Energy The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. The term activation energy is also used for an. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. It is equal to the difference between the. Activation energy plays a crucial role in. Is The Difference Between Threshold Energy And Activation Energy.
From socratic.org
What is activation energy? What is threshold energy? What are the Is The Difference Between Threshold Energy And Activation Energy The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. It is equal to the difference between the. In other words, the activation energy is what. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. The difference between the. Is The Difference Between Threshold Energy And Activation Energy.
From professional.patrickneyman.com
Activation Energy Equation Is The Difference Between Threshold Energy And Activation Energy The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. The term activation energy is also used for an. The difference between the threshold energy and whatever internal energy molecules have is. Is The Difference Between Threshold Energy And Activation Energy.
From www.toppr.com
The energy of activation for the backward reaction is Is The Difference Between Threshold Energy And Activation Energy Activation energy plays a crucial role in determining the rate of a chemical reaction; In other words, the activation energy is what. Lower activation energy means faster reaction. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The difference between the threshold energy and whatever internal energy molecules have is. Is The Difference Between Threshold Energy And Activation Energy.
From www.youtube.com
Difference between Activation energy and Threshold energy YouTube Is The Difference Between Threshold Energy And Activation Energy The difference between the quantities is briefly outlined in iupac green book [1, pp. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. It is the minimum. Is The Difference Between Threshold Energy And Activation Energy.
From fixportillobadgered.z21.web.core.windows.net
Energy Diagram Activation Energy Is The Difference Between Threshold Energy And Activation Energy The term activation energy is also used for an. It is equal to the difference between the. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. The difference between the quantities. Is The Difference Between Threshold Energy And Activation Energy.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Is The Difference Between Threshold Energy And Activation Energy The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. Activation energy plays a crucial role in determining the rate of a chemical reaction; Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The difference between the. Is The Difference Between Threshold Energy And Activation Energy.
From byjus.com
The forward and backward activation energy of exothermic reaction A→ B Is The Difference Between Threshold Energy And Activation Energy The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. Lower activation energy means faster reaction. The difference between the quantities is briefly outlined in iupac green book [1, pp. In other words, the activation energy is what. Activation energy plays a crucial role in determining the rate of a chemical reaction; Activation. Is The Difference Between Threshold Energy And Activation Energy.
From byjus.com
What happens when the energy of a reaction if more than threshold Is The Difference Between Threshold Energy And Activation Energy The difference between the quantities is briefly outlined in iupac green book [1, pp. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. Lower activation energy means faster reaction. Activation energy plays a crucial role in determining the rate of a chemical reaction; The term activation energy is also used. Is The Difference Between Threshold Energy And Activation Energy.
From edurev.in
Difference between activation energy and threshold energy I know the Is The Difference Between Threshold Energy And Activation Energy The term that refers to the difference between the energy of the transition state and the energy of the reactants is activation energy. Activation energy plays a crucial role in determining the rate of a chemical reaction; It is equal to the difference between the. The term activation energy is also used for an. The difference between the threshold energy. Is The Difference Between Threshold Energy And Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Is The Difference Between Threshold Energy And Activation Energy It is equal to the difference between the. The term activation energy is also used for an. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. The difference between the quantities is briefly outlined in iupac green book [1, pp. Lower activation energy means faster reaction. In other words, the activation energy. Is The Difference Between Threshold Energy And Activation Energy.
From www.youtube.com
R2.2.4 Activation energy YouTube Is The Difference Between Threshold Energy And Activation Energy The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. Activation energy plays a crucial role in determining the rate of a chemical reaction; The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy ea. The term that refers to the difference between the energy. Is The Difference Between Threshold Energy And Activation Energy.
From byjus.com
26. What is difference between threshold energy and activation energy? Is The Difference Between Threshold Energy And Activation Energy Lower activation energy means faster reaction. The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. The term activation energy is also used for an. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. Activation energy is primarily associated with chemical reactions, where. Is The Difference Between Threshold Energy And Activation Energy.
From www.britannica.com
Activation energy Definition & Facts Britannica Is The Difference Between Threshold Energy And Activation Energy The term activation energy is also used for an. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. Activation energy plays a crucial role in determining the rate of a chemical reaction; The difference between the quantities is briefly outlined in iupac green book [1, pp. Lower activation energy means. Is The Difference Between Threshold Energy And Activation Energy.
From www.differencebetween.com
Difference Between Energy and Activation Energy Compare the Is The Difference Between Threshold Energy And Activation Energy Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The difference between the quantities is briefly outlined in iupac green book [1, pp. The term activation energy is also used for an. The excess energy which must be supplied to the reactants to undergo chemical reactions is called activation energy. Is The Difference Between Threshold Energy And Activation Energy.
From www.youtube.com
Relationship between activation energy and reaction rate Reaction Is The Difference Between Threshold Energy And Activation Energy Activation energy plays a crucial role in determining the rate of a chemical reaction; The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. It is equal to the difference between the. The term that refers to the difference between the energy of the transition state and the energy of the reactants is. Is The Difference Between Threshold Energy And Activation Energy.
From byjus.com
What happens when the energy of a reaction if more than threshold Is The Difference Between Threshold Energy And Activation Energy The difference between the threshold energy and whatever internal energy molecules have is called the activation energy. It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming. Activation energy is primarily associated with chemical reactions, where it represents the energy required for reactants to transform into. The term that refers to. Is The Difference Between Threshold Energy And Activation Energy.