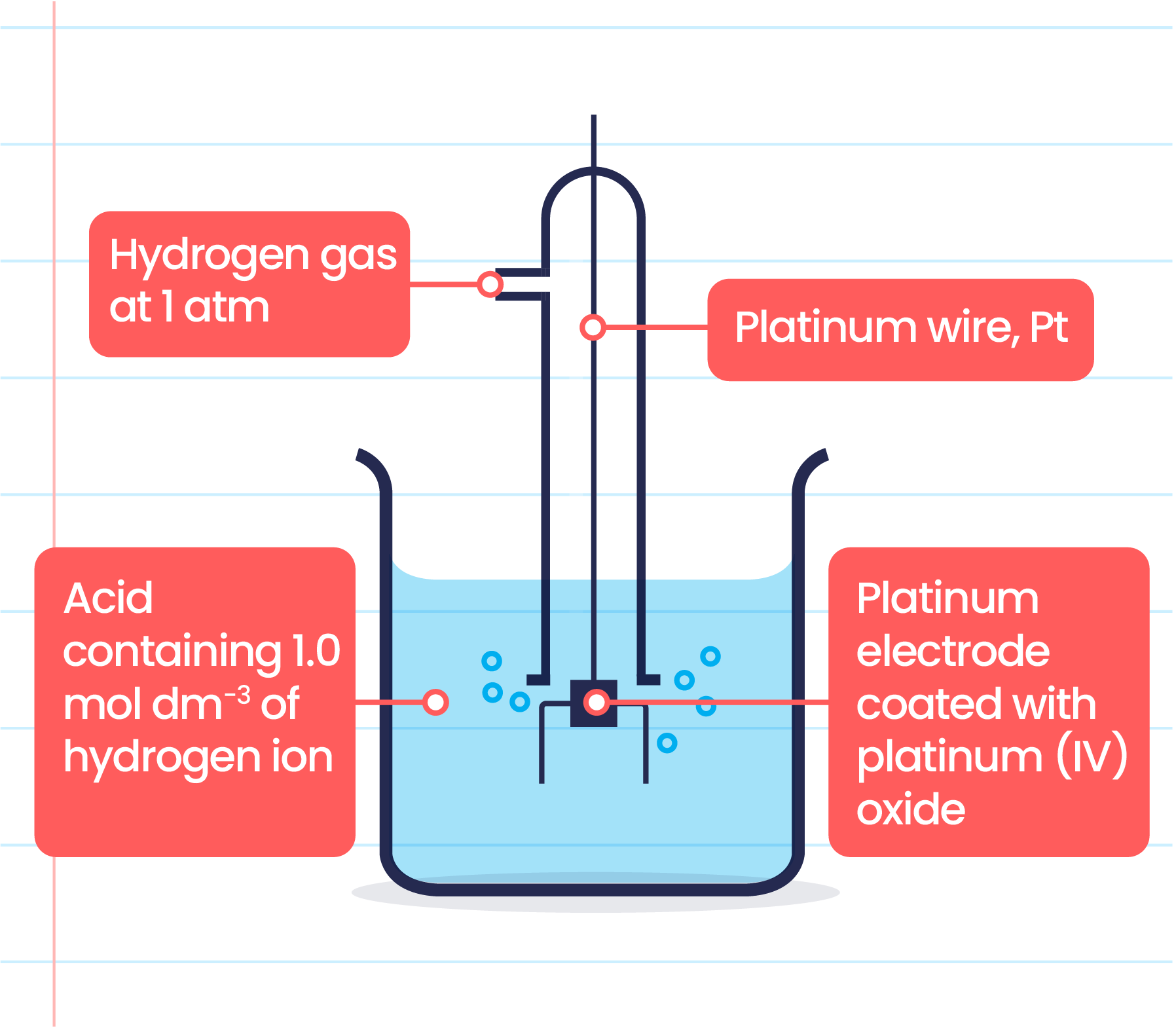
Standard Hydrogen Electrode Potential Table . The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 45 rows standard electrode potentials in aqueous solution at 25°c. The structure of the standard hydrogen electrode is.
from question.pandai.org
Wikipedia) t0 = 298.15 k (25.00°c) p0. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. Values relative to the standard hydrogen electrode (she). The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:.
Standard Electrode Potential
Standard Hydrogen Electrode Potential Table The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Values relative to the standard hydrogen electrode (she). 45 rows standard electrode potentials in aqueous solution at 25°c. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The structure of the standard hydrogen electrode is. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Wikipedia) t0 = 298.15 k (25.00°c) p0.
From www.researchgate.net
Absolute and relative (respect to Standard Hydrogen Electrode, SHE Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. 45 rows standard electrode potentials in aqueous solution at 25°c. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The potential. Standard Hydrogen Electrode Potential Table.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Values relative to the standard hydrogen electrode (she). The larger the value of the standard reduction potential, the easier it is for. Standard Hydrogen Electrode Potential Table.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Standard Hydrogen Electrode Potential Table Values relative to the standard hydrogen electrode (she). The structure of the standard hydrogen electrode is. Wikipedia) t0 = 298.15 k (25.00°c) p0. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 45 rows standard electrode potentials in aqueous solution at 25°c. The larger the value of the standard reduction potential, the easier. Standard Hydrogen Electrode Potential Table.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 45 rows standard electrode potentials in aqueous solution at 25°c. The structure of the standard hydrogen electrode is. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard. Standard Hydrogen Electrode Potential Table.
From users.highland.edu
Standard Potentials Standard Hydrogen Electrode Potential Table The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard. Standard Hydrogen Electrode Potential Table.
From mungfali.com
Table Of Standard Electrode Potentials Standard Hydrogen Electrode Potential Table The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Values relative to. Standard Hydrogen Electrode Potential Table.
From mungfali.com
Standard Electrode Potential Table Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative to the standard hydrogen electrode (she). The structure of the standard hydrogen electrode is. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The larger the value of the standard reduction potential, the easier it is for the element. Standard Hydrogen Electrode Potential Table.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Potential Table 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Wikipedia) t0 = 298.15 k (25.00°c) p0. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen. Standard Hydrogen Electrode Potential Table.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Hydrogen Electrode Potential Table The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. The structure of the standard hydrogen electrode is. The. Standard Hydrogen Electrode Potential Table.
From ar.inspiredpencil.com
Electro Potential Table Standard Hydrogen Electrode Potential Table The structure of the standard hydrogen electrode is. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. Values relative to the standard hydrogen electrode (she). The potential of. Standard Hydrogen Electrode Potential Table.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Hydrogen Electrode Potential Table The structure of the standard hydrogen electrode is. Wikipedia) t0 = 298.15 k (25.00°c) p0. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:.. Standard Hydrogen Electrode Potential Table.
From gaskatel.de
Reference electrode Gaskatel Standard Hydrogen Electrode Potential Table Values relative to the standard hydrogen electrode (she). The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Wikipedia) t0 = 298.15 k (25.00°c) p0. 45 rows standard electrode potentials in aqueous solution at 25°c. The structure of the standard hydrogen electrode is. 372 rows the data below tabulates standard electrode potentials (e °),. Standard Hydrogen Electrode Potential Table.
From www.researchgate.net
Comparison of the voltage vs. standard hydrogen electrode (SHE Standard Hydrogen Electrode Potential Table The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is. Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:.. Standard Hydrogen Electrode Potential Table.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Standard Hydrogen Electrode Potential Table The structure of the standard hydrogen electrode is. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 45 rows standard electrode potentials in aqueous solution at 25°c. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Wikipedia) t0 = 298.15 k (25.00°c). Standard Hydrogen Electrode Potential Table.
From ncerthelp.blogspot.com
NCERT Solutions, CBSE Sample Papers and Syllabus for Class 9 to 12 Standard Hydrogen Electrode Potential Table 45 rows standard electrode potentials in aqueous solution at 25°c. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. Values relative to the standard hydrogen electrode (she). The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Wikipedia) t0 = 298.15 k (25.00°c). Standard Hydrogen Electrode Potential Table.
From www.researchgate.net
Electrochemical reaction standard potentials (E0, V vs standard Standard Hydrogen Electrode Potential Table 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative to the standard hydrogen. Standard Hydrogen Electrode Potential Table.
From www.pveducation.org
Standard Potential PVEducation Standard Hydrogen Electrode Potential Table The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is. 45 rows standard electrode potentials in aqueous solution at 25°c. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates. Standard Hydrogen Electrode Potential Table.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Hydrogen Electrode Potential Table Values relative to the standard hydrogen electrode (she). 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. The. Standard Hydrogen Electrode Potential Table.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Standard Hydrogen Electrode Potential Table 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen electrode (she). The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Wikipedia) t0 = 298.15 k. Standard Hydrogen Electrode Potential Table.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Hydrogen Electrode Potential Table The structure of the standard hydrogen electrode is. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 45 rows standard electrode potentials in aqueous solution at 25°c. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Wikipedia) t0 = 298.15 k. Standard Hydrogen Electrode Potential Table.
From pubs.acs.org
Absolute Potential of the Standard Hydrogen Electrode and the Problem Standard Hydrogen Electrode Potential Table 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The structure of the standard hydrogen electrode is. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Values relative to the standard hydrogen electrode (she). The larger the value of the standard. Standard Hydrogen Electrode Potential Table.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Hydrogen Electrode Potential Table The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 45 rows standard electrode potentials in aqueous solution at 25°c. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is. Wikipedia) t0 = 298.15 k (25.00°c). Standard Hydrogen Electrode Potential Table.
From pandai.me
Standard Electrode Potential Standard Hydrogen Electrode Potential Table Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 45 rows standard electrode potentials in aqueous solution at 25°c. The. Standard Hydrogen Electrode Potential Table.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. Values relative to the standard hydrogen electrode (she). The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 45 rows standard electrode potentials in aqueous solution at. Standard Hydrogen Electrode Potential Table.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Potential Table 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The structure of the standard hydrogen electrode is. 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen electrode (she). Wikipedia) t0 = 298.15 k (25.00°c) p0. The potential of the standard hydrogen. Standard Hydrogen Electrode Potential Table.
From parteybosa.blogspot.com
Standard Electrode Potential Table JEE Main Electrochemistry Part4 Standard Hydrogen Electrode Potential Table The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the. Standard Hydrogen Electrode Potential Table.
From www.pinterest.com
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Hydrogen Electrode Potential Table The structure of the standard hydrogen electrode is. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Wikipedia) t0 = 298.15 k (25.00°c) p0. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. Values relative to the. Standard Hydrogen Electrode Potential Table.
From saylordotorg.github.io
Standard Potentials Standard Hydrogen Electrode Potential Table Values relative to the standard hydrogen electrode (she). The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. The structure of the standard hydrogen electrode is. 45 rows standard electrode potentials in aqueous solution. Standard Hydrogen Electrode Potential Table.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The structure of the standard hydrogen electrode is. 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen electrode (she). 372 rows the data below tabulates standard electrode potentials (e °),. Standard Hydrogen Electrode Potential Table.
From youtube.com
19.1.4 Predict whether a reaction will be spontaneous using standard Standard Hydrogen Electrode Potential Table 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen electrode (she). Wikipedia) t0 = 298.15 k (25.00°c) p0. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to. Standard Hydrogen Electrode Potential Table.
From www.chemistryland.com
Lab 8 Single Replacement Reactions Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. The structure of the standard hydrogen electrode is. The larger the value of the standard reduction potential, the easier it is for the element to be reduced (gain. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. Values relative to the. Standard Hydrogen Electrode Potential Table.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Hydrogen Electrode Potential Table The structure of the standard hydrogen electrode is. Values relative to the standard hydrogen electrode (she). The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The larger the value of the standard. Standard Hydrogen Electrode Potential Table.
From www.researchgate.net
Reversible Potentials for Common Electrode Materials at 25 8C a Standard Hydrogen Electrode Potential Table The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. 45 rows standard electrode potentials in aqueous solution at 25°c. The structure of the standard hydrogen electrode is. Wikipedia) t0 = 298.15 k. Standard Hydrogen Electrode Potential Table.
From www.researchgate.net
4 Potential relation between the experimentally applied Standard Standard Hydrogen Electrode Potential Table Wikipedia) t0 = 298.15 k (25.00°c) p0. Values relative to the standard hydrogen electrode (she). 45 rows standard electrode potentials in aqueous solution at 25°c. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The larger the value of the standard reduction potential, the easier it is for the element to be reduced. Standard Hydrogen Electrode Potential Table.
From www.toppr.com
Using the standard electrode potentials Standard Hydrogen Electrode Potential Table 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 45 rows standard electrode potentials in aqueous solution at 25°c. Values relative to the standard hydrogen electrode (she). The structure of the standard. Standard Hydrogen Electrode Potential Table.