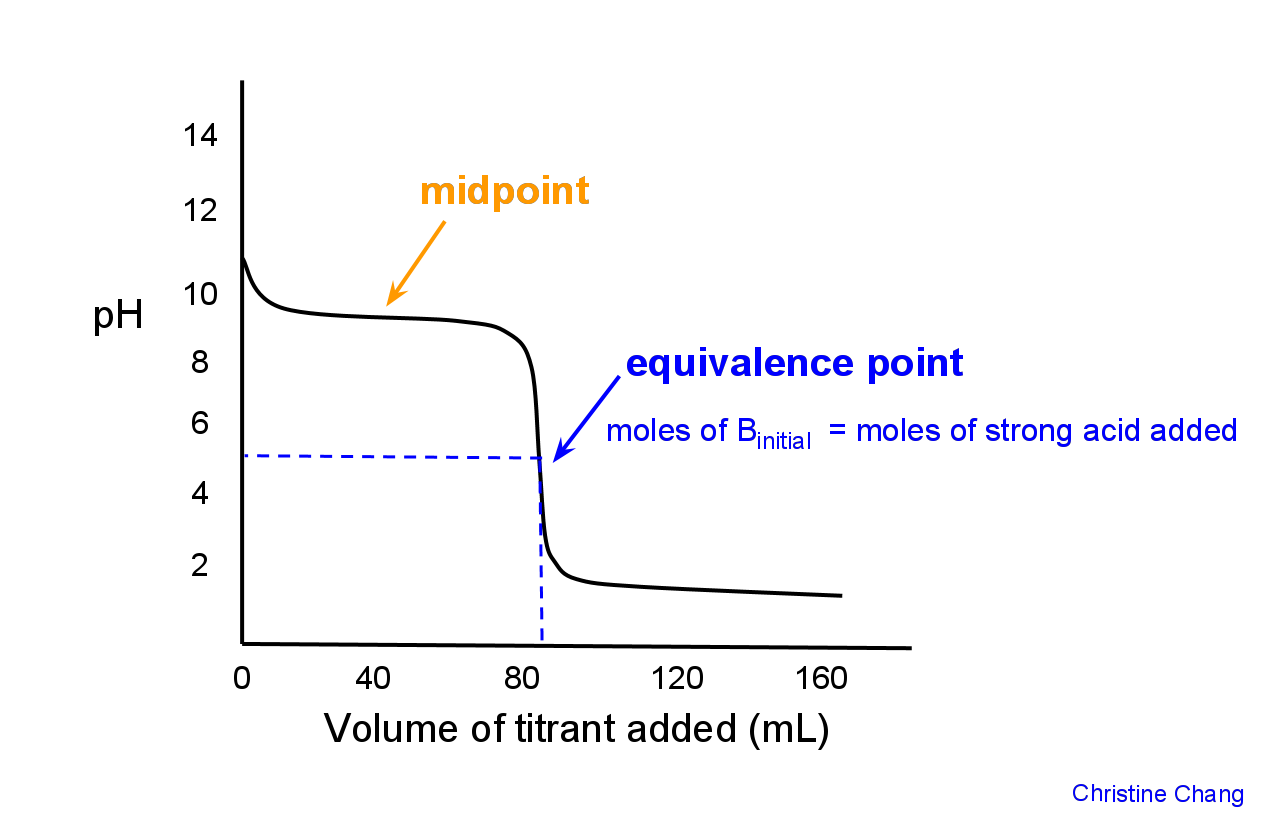
Indicator Used In Strong Acid Strong Base Titration . This page assumes that you know. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Therefore, you would want an. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 2) the equivalence point is at ph 7 because it is. Calculating ph for titration solutions: 1) the ph changes slowly in the first half of the titration. Any of the three indicators will exhibit.
from chem.libretexts.org
This page assumes that you know. 2) the equivalence point is at ph 7 because it is. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 1) the ph changes slowly in the first half of the titration. Therefore, you would want an. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Any of the three indicators will exhibit. Calculating ph for titration solutions:
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Indicator Used In Strong Acid Strong Base Titration 2) the equivalence point is at ph 7 because it is. Any of the three indicators will exhibit. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. Calculating ph for titration solutions: 1) the ph changes slowly in the first half of the titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Therefore, you would want an. 2) the equivalence point is at ph 7 because it is. This page assumes that you know.
From mungfali.com
Acid Base Titration Indicator Indicator Used In Strong Acid Strong Base Titration 2) the equivalence point is at ph 7 because it is. Calculating ph for titration solutions: This page assumes that you know. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 1) the ph changes slowly in the first half. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Indicator Used In Strong Acid Strong Base Titration This page assumes that you know. Therefore, you would want an. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 2) the equivalence point is. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Indicator Used In Strong Acid Strong Base Titration Therefore, you would want an. 1) the ph changes slowly in the first half of the titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. This page assumes that you know. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph. Indicator Used In Strong Acid Strong Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Any of the three indicators will exhibit. This page assumes that you know. 1) the ph changes slowly in the first half of the titration. Calculating ph for titration solutions: 2) the equivalence point is at ph 7 because it is. In the titration of. Indicator Used In Strong Acid Strong Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator Used In Strong Acid Strong Base Titration In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 1) the ph changes slowly in the first half of the titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. 2) the equivalence point. Indicator Used In Strong Acid Strong Base Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Indicator Used In Strong Acid Strong Base Titration In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 2) the equivalence point is at ph 7 because it is. Any of the three indicators will exhibit. This page assumes that you know. Calculating ph for titration solutions: A titration. Indicator Used In Strong Acid Strong Base Titration.
From saylordotorg.github.io
AcidBase Titrations Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Any of the three indicators will exhibit. This page assumes that you know. 1) the ph changes slowly in the first half of the titration. Calculating ph for titration solutions: 2) the equivalence point is at ph 7 because it is. Therefore, you would want. Indicator Used In Strong Acid Strong Base Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Indicator Used In Strong Acid Strong Base Titration This page assumes that you know. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. Therefore, you would want an. Calculating ph for titration solutions: Any of the three indicators will exhibit. 1) the ph changes slowly in the first. Indicator Used In Strong Acid Strong Base Titration.
From saylordotorg.github.io
AcidBase Titrations Indicator Used In Strong Acid Strong Base Titration Calculating ph for titration solutions: Any of the three indicators will exhibit. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. 1) the ph changes slowly in the first half of the titration. This page assumes that you know. Therefore, you would want an. In the titration of a weak acid with a strong. Indicator Used In Strong Acid Strong Base Titration.
From exytfczfn.blob.core.windows.net
What Is The Purpose Of An AcidBase Titration at Adolph Tilton blog Indicator Used In Strong Acid Strong Base Titration Any of the three indicators will exhibit. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 1) the ph changes slowly in the first half. Indicator Used In Strong Acid Strong Base Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. This page assumes that you know. 2) the equivalence point is at ph 7 because it. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 Indicator Used In Strong Acid Strong Base Titration Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. Therefore, you would want an. This page assumes that you know.. Indicator Used In Strong Acid Strong Base Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator Used In Strong Acid Strong Base Titration 2) the equivalence point is at ph 7 because it is. Calculating ph for titration solutions: Therefore, you would want an. 1) the ph changes slowly in the first half of the titration. Any of the three indicators will exhibit. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6776086 Indicator Used In Strong Acid Strong Base Titration This page assumes that you know. 2) the equivalence point is at ph 7 because it is. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: Therefore, you would want an. Any of the three indicators will exhibit. 1) the ph changes slowly in the first half of the titration. 2) the equivalence point is at ph 7 because it is. This page assumes that you. Indicator Used In Strong Acid Strong Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Any of the three indicators will exhibit. 2) the equivalence point is at ph 7 because it is. Calculating ph for titration solutions: 1) the ph changes slowly in the first half of the titration. Therefore, you would want an. This page assumes that you. Indicator Used In Strong Acid Strong Base Titration.
From philschatz.com
AcidBase Titrations · Chemistry Indicator Used In Strong Acid Strong Base Titration This page assumes that you know. Calculating ph for titration solutions: Therefore, you would want an. 1) the ph changes slowly in the first half of the titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Any of the three indicators will exhibit. In the titration of a weak acid with a strong. Indicator Used In Strong Acid Strong Base Titration.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Indicator Used In Strong Acid Strong Base Titration Therefore, you would want an. Calculating ph for titration solutions: This page assumes that you know. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7.. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator Used In Strong Acid Strong Base Titration This page assumes that you know. Any of the three indicators will exhibit. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. Therefore, you would want an. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Indicator Used In Strong Acid Strong Base Titration.
From www.edukate.me
Indicator is Used in AcidBase Titration Using a phenolphthalein Indicator Used In Strong Acid Strong Base Titration 2) the equivalence point is at ph 7 because it is. Therefore, you would want an. 1) the ph changes slowly in the first half of the titration. This page assumes that you know. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. In the titration of a weak acid with a strong base,. Indicator Used In Strong Acid Strong Base Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Indicator Used In Strong Acid Strong Base Titration 2) the equivalence point is at ph 7 because it is. Any of the three indicators will exhibit. Calculating ph for titration solutions: Therefore, you would want an. This page assumes that you know. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Indicator Used In Strong Acid Strong Base Titration In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 1) the ph changes slowly in the first half of the titration. Calculating ph for titration solutions: Therefore, you would want an. This page assumes that you know. 2) the equivalence. Indicator Used In Strong Acid Strong Base Titration.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 1) the ph changes slowly in the first half of the. Indicator Used In Strong Acid Strong Base Titration.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Indicator Used In Strong Acid Strong Base Titration In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. This page assumes that you know. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. 1) the ph changes slowly in the first half of. Indicator Used In Strong Acid Strong Base Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicator Used In Strong Acid Strong Base Titration Any of the three indicators will exhibit. 2) the equivalence point is at ph 7 because it is. This page assumes that you know. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. 1) the ph changes slowly in the first half of the titration. Calculating ph for titration solutions: Therefore, you would want. Indicator Used In Strong Acid Strong Base Titration.
From www.sliderbase.com
Titration Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. This page assumes that you know. 1) the ph changes slowly in the first half of the titration. Calculating ph for titration solutions: Therefore, you would want an. Any of the three indicators will exhibit. In the titration of a weak acid with a strong. Indicator Used In Strong Acid Strong Base Titration.
From www.youtube.com
To study the pH change in the titration of strong base and strong acid Indicator Used In Strong Acid Strong Base Titration Therefore, you would want an. Any of the three indicators will exhibit. 1) the ph changes slowly in the first half of the titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. This page assumes that you know. 2) the equivalence point is at ph 7 because it is. Calculating ph for titration. Indicator Used In Strong Acid Strong Base Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Indicator Used In Strong Acid Strong Base Titration Calculating ph for titration solutions: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. 1) the ph changes slowly in the first half of the titration. Therefore, you would want an. 2) the equivalence point is at ph 7 because it is. In the titration of a weak acid with a strong base, the. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Indicator Used In Strong Acid Strong Base Titration In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. Calculating ph for titration solutions: 2) the equivalence point is at ph 7 because it is. Any of the three indicators will exhibit. This page assumes that you know. 1) the. Indicator Used In Strong Acid Strong Base Titration.
From byjus.com
Study The pH Change In The Titration Of A Strong Base Using Universal Indicator Used In Strong Acid Strong Base Titration In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Calculating ph for titration solutions: Any of the three indicators will exhibit. Therefore, you would want. Indicator Used In Strong Acid Strong Base Titration.
From exyktdgef.blob.core.windows.net
The Indicator Used In Acid Base Titration Are at Paul Guzman blog Indicator Used In Strong Acid Strong Base Titration This page assumes that you know. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. 1) the ph changes slowly in the first half of the titration. Any of the three indicators will exhibit. Calculating ph for titration solutions: 2). Indicator Used In Strong Acid Strong Base Titration.
From loeoavwmr.blob.core.windows.net
Selecting Indicators For AcidBase Titrations Lab at Teresa Colwell blog Indicator Used In Strong Acid Strong Base Titration 1) the ph changes slowly in the first half of the titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. Therefore, you would want an. This page assumes that you know. 2) the equivalence point is at ph 7 because it is. Any of the three indicators will exhibit. Calculating ph for titration. Indicator Used In Strong Acid Strong Base Titration.
From dxonbtcto.blob.core.windows.net
Chemical Indicator AcidBase Titration at Hilda Johnston blog Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. This page assumes that you know. Any of the three indicators will exhibit. Calculating ph for titration solutions: 1) the ph changes slowly in the first half of the titration. Therefore, you would want an. 2) the equivalence point is at ph 7 because it. Indicator Used In Strong Acid Strong Base Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Indicator Used In Strong Acid Strong Base Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. 2) the equivalence point is at ph 7 because it is. Calculating ph for titration solutions: This page assumes that you know. Therefore, you would want an. In the titration of a weak acid with a strong base, the conjugate base of the weak acid. Indicator Used In Strong Acid Strong Base Titration.
From www.slideshare.net
Acid base titration Indicator Used In Strong Acid Strong Base Titration Therefore, you would want an. In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the ph at the equivalence point greater than 7. This page assumes that you know. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with. 1) the ph changes slowly. Indicator Used In Strong Acid Strong Base Titration.