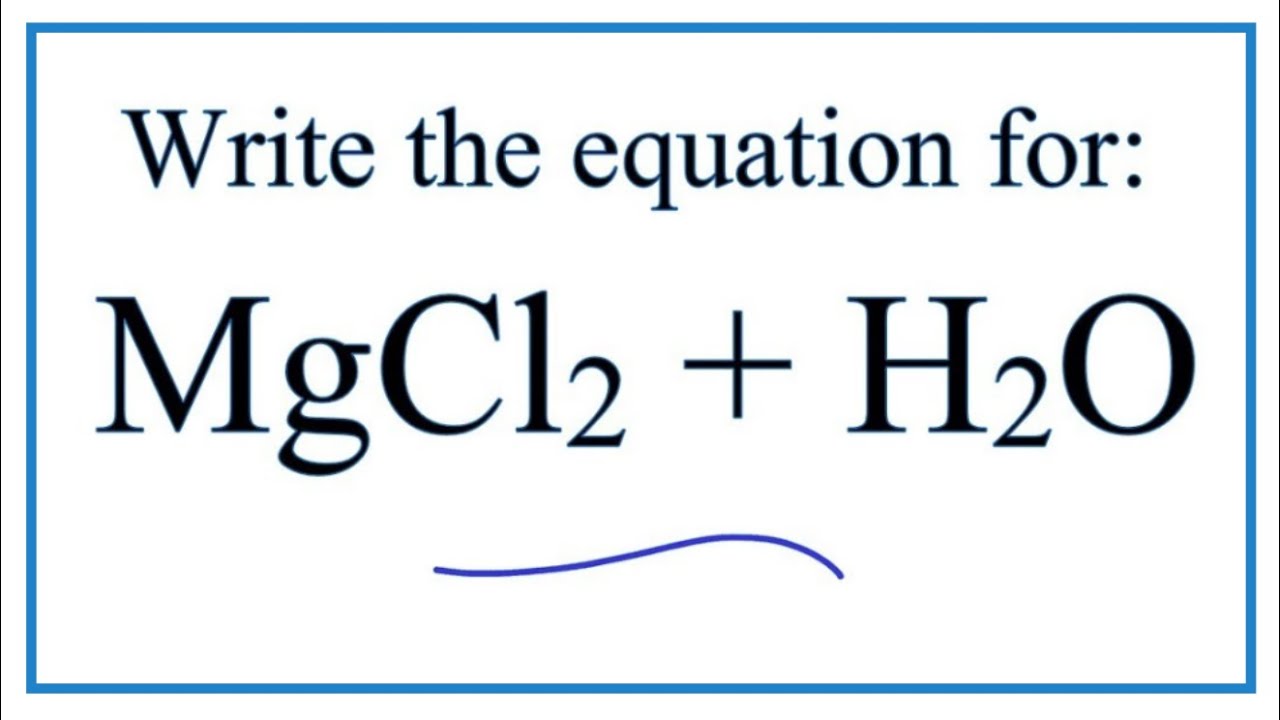Magnesium Chloride Mass Equation . The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The chemical formula for magnesium chloride is mgcl2. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions.
from www.youtube.com
The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The chemical formula for magnesium chloride is mgcl2. It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions.
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube
Magnesium Chloride Mass Equation 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. It is an ionic compound, composed. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The chemical formula for magnesium chloride is mgcl2.
From www.toppr.com
Magnesium combines with chlorine to form magnesium chloride. The Magnesium Chloride Mass Equation The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. It is an ionic compound, composed. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The. Magnesium Chloride Mass Equation.
From avopix.com
Magnesium Chloride Properties and Chemical Royalty Free Stock Vector Magnesium Chloride Mass Equation Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. It is an ionic compound, composed. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first. Magnesium Chloride Mass Equation.
From krystal-kkim.blogspot.com
What Is the Mass Percent of Magnesium in Magnesium Chloride Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. It is an ionic. Magnesium Chloride Mass Equation.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Chloride Mass Equation 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. The chemical formula for magnesium chloride is mgcl2. At the heart of magnesium chloride’s usefulness is its unique set of chemical and. Magnesium Chloride Mass Equation.
From ask.modifiyegaraj.com
Molar Mass Of Magnesium Chloride Asking List Magnesium Chloride Mass Equation The chemical formula for magnesium chloride is mgcl2. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s usefulness is its unique set of. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED Question 4 Magnesium chloride combines with potassium yielding Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED Magnesium reacts with hydrochloric acid, HCl, to form magnesium Magnesium Chloride Mass Equation It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The chemical formula for magnesium chloride is mgcl2. The first step to finding the molar mass of magnesium chloride is to count the. Magnesium Chloride Mass Equation.
From www.chegg.com
Solved Complete the table below for calculating the molar Magnesium Chloride Mass Equation 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The chemical formula for magnesium chloride is mgcl2. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single. Magnesium Chloride Mass Equation.
From selfdirectedce.com
How to Write the Net Ionic Equation for Mg + CuCl2 = MgCl2 + Cu Magnesium Chloride Mass Equation Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. It is an ionic compound, composed. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first. Magnesium Chloride Mass Equation.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride Mass Equation It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The first. Magnesium Chloride Mass Equation.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride Mass Equation Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. It is an ionic compound, composed. The chemical formula for magnesium chloride is mgcl2. The first step to finding the molar mass of magnesium chloride is to count the number of each. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Magnesium Chloride Mass Equation It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single. Magnesium Chloride Mass Equation.
From www.bartleby.com
Answered Magnesium metal reacts with… bartleby Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. The chemical formula for. Magnesium Chloride Mass Equation.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Mass Equation 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The chemical formula for magnesium chloride is mgcl2. The first step to finding the molar mass. Magnesium Chloride Mass Equation.
From www.slideserve.com
PPT Mg(OH) 2 + 2HCl → 2H 2 O + MgCl 2 PowerPoint Presentation, free Magnesium Chloride Mass Equation It is an ionic compound, composed. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first. Magnesium Chloride Mass Equation.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630 Magnesium Chloride Mass Equation Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The chemical formula for. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED EXPERIMENT 2 Perform the calculations and record the following Magnesium Chloride Mass Equation 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. It is an ionic compound, composed. The chemical formula for magnesium chloride is mgcl2. Magnesium chloride (mgcl₂) is an ionic compound formed. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrogen chloride gas to form Magnesium Chloride Mass Equation 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. It is an ionic compound, composed.. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED Experiment Determination of an Empirical Formula Report Sheet Magnesium Chloride Mass Equation It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The first. Magnesium Chloride Mass Equation.
From www.youtube.com
Is MgCl2 (Magnesium chloride) Ionic or Covalent? YouTube Magnesium Chloride Mass Equation 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The chemical formula for magnesium chloride is mgcl2. It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The first. Magnesium Chloride Mass Equation.
From snipe.fm
😍 Empirical formula of magnesium chloride. ChemTeam Determine the Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. 95.211 g/mol anhydrous, and. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrochloric acid to produce Magnesium Chloride Mass Equation It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The chemical formula for magnesium chloride is mgcl2. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The first step to finding the molar mass of magnesium chloride is to count the. Magnesium Chloride Mass Equation.
From jefferson-kmcclain.blogspot.com
What Is the Mass Percent of Mg in Magnesium Chloride Magnesium Chloride Mass Equation The chemical formula for magnesium chloride is mgcl2. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. At the heart of magnesium chloride’s usefulness is its unique set of chemical and. Magnesium Chloride Mass Equation.
From www.youtube.com
Write the chemical formula of the following compounds (a)Magnesium Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnesium chloride (mgcl₂) is an ionic. Magnesium Chloride Mass Equation.
From www.youtube.com
Empirical Formula of Magnesium Chloride LAB YouTube Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. It is an ionic compound, composed. The first. Magnesium Chloride Mass Equation.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science hive Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The chemical formula for magnesium chloride is mgcl2. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The first step to finding the molar mass. Magnesium Chloride Mass Equation.
From husnahuzepha.blogspot.com
51+ calculate the molecular mass of magnesium chloride HusnaHuzepha Magnesium Chloride Mass Equation The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 95.211 g/mol anhydrous, and. Magnesium Chloride Mass Equation.
From klaldmnft.blob.core.windows.net
Sodium Carbonate And Magnesium Chloride Formula at Michael Knutson blog Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. It is an ionic compound, composed. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine. Magnesium Chloride Mass Equation.
From www.numerade.com
SOLVED A solution is prepared by dissolving 25 grams of magnesium Magnesium Chloride Mass Equation The chemical formula for magnesium chloride is mgcl2. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. At the heart of magnesium chloride’s usefulness is its unique set of. Magnesium Chloride Mass Equation.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium Chloride Mass Equation Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. It is an ionic compound, composed. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The first. Magnesium Chloride Mass Equation.
From brainly.in
What is the mass of Magnesium chloride required to prepare 500 cm³ of a Magnesium Chloride Mass Equation The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. It is an ionic compound, composed. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine. Magnesium Chloride Mass Equation.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Magnesium Chloride Mass Equation Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. It is an ionic compound, composed. The first step to finding the molar mass of magnesium chloride is to count the number of each atom present in a single molecule using. At the heart of magnesium chloride’s usefulness is its unique set of chemical and. Magnesium Chloride Mass Equation.
From jbdizkkrki.blogspot.com
Magnesium Chloride Formula Unit The Structure Of Matter Section 1 Magnesium Chloride Mass Equation At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. The chemical formula for magnesium chloride is mgcl2. It is an ionic compound, composed. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The first step to finding the molar mass of magnesium chloride is to count the number of. Magnesium Chloride Mass Equation.
From www.showme.com
ShowMe magnesium chloride Magnesium Chloride Mass Equation Magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The chemical formula for magnesium chloride is mgcl2. At the heart of magnesium chloride’s usefulness is its unique set of chemical and physical properties. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. It is an ionic compound, composed. The first. Magnesium Chloride Mass Equation.