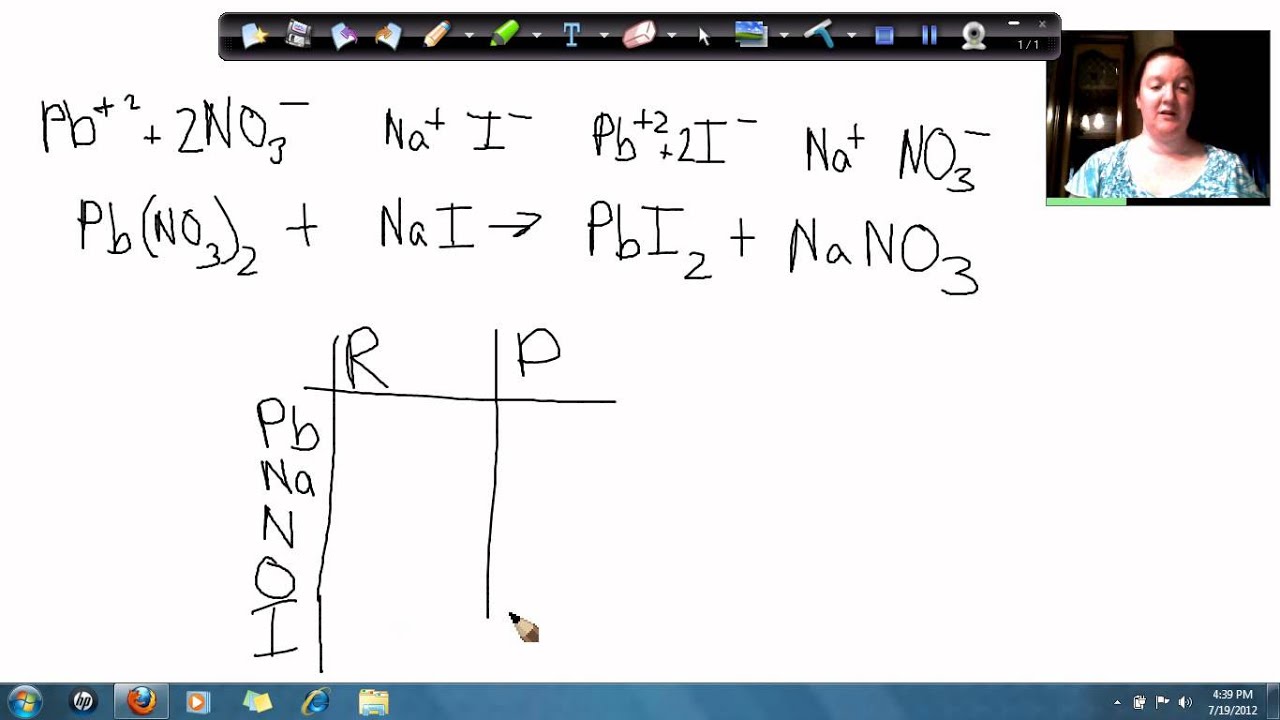
Lead Nitrate And Sodium Hydroxide Formula . First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). which solution could be used to precipitate the barium ion, ba 2+, in a water sample: this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. it is a chemical compound with the formula pb (no₃)₂. The balanced equation will be calculated along. It consists of lead and nitrate ions and appears as a colorless or white. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Sodium chloride, sodium hydroxide, or. enter an equation of an ionic chemical equation and press the balance button.
from exoncvmev.blob.core.windows.net
enter an equation of an ionic chemical equation and press the balance button. First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. It consists of lead and nitrate ions and appears as a colorless or white. Sodium chloride, sodium hydroxide, or. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. The balanced equation will be calculated along. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. it is a chemical compound with the formula pb (no₃)₂. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii).
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons
Lead Nitrate And Sodium Hydroxide Formula The balanced equation will be calculated along. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. The balanced equation will be calculated along. it is a chemical compound with the formula pb (no₃)₂. It consists of lead and nitrate ions and appears as a colorless or white. Sodium chloride, sodium hydroxide, or. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. enter an equation of an ionic chemical equation and press the balance button. which solution could be used to precipitate the barium ion, ba 2+, in a water sample:
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead Nitrate And Sodium Hydroxide Formula it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: First, we need. Lead Nitrate And Sodium Hydroxide Formula.
From www.numerade.com
SOLVEDand net ionic equation for the following eactions Write Lead Nitrate And Sodium Hydroxide Formula The balanced equation will be calculated along. it is a chemical compound with the formula pb (no₃)₂. Sodium chloride, sodium hydroxide, or. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. It consists of lead and nitrate ions and appears as a colorless or white. this page looks. Lead Nitrate And Sodium Hydroxide Formula.
From www.slideshare.net
Precipitation react2 Lead Nitrate And Sodium Hydroxide Formula which solution could be used to precipitate the barium ion, ba 2+, in a water sample: First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). It consists of lead and nitrate ions and. Lead Nitrate And Sodium Hydroxide Formula.
From dxoezqcmu.blob.core.windows.net
Lead Hydroxide Ionic Formula at Clara Lott blog Lead Nitrate And Sodium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. First, we need to write the. Lead Nitrate And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED the word equation for each precipitation reaction. Determine Lead Nitrate And Sodium Hydroxide Formula It consists of lead and nitrate ions and appears as a colorless or white. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it is a chemical compound with the formula pb (no₃)₂. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Nitrate And Sodium Hydroxide Formula.
From www.tessshebaylo.com
Chemical Equation For Water And Sodium Chloride Tessshebaylo Lead Nitrate And Sodium Hydroxide Formula it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). It consists of lead and nitrate ions and appears as a colorless or white. First, we need to write the molecular equation. Lead Nitrate And Sodium Hydroxide Formula.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead Nitrate And Sodium Hydroxide Formula it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. enter an equation of an ionic chemical equation and press the balance button. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it is a chemical compound with the formula pb. Lead Nitrate And Sodium Hydroxide Formula.
From dxoezqcmu.blob.core.windows.net
Lead Hydroxide Ionic Formula at Clara Lott blog Lead Nitrate And Sodium Hydroxide Formula which solution could be used to precipitate the barium ion, ba 2+, in a water sample: It consists of lead and nitrate ions and appears as a colorless or white. enter an equation of an ionic chemical equation and press the balance button. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather. Lead Nitrate And Sodium Hydroxide Formula.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Nitrate And Sodium Hydroxide Formula Sodium chloride, sodium hydroxide, or. It consists of lead and nitrate ions and appears as a colorless or white. The balanced equation will be calculated along. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. it is a chemical compound with the formula pb (no₃)₂. which. Lead Nitrate And Sodium Hydroxide Formula.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead Nitrate And Sodium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. Sodium chloride, sodium hydroxide, or. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It consists of lead and nitrate ions and appears as a colorless or white. lead(ii) ion reacts with aqueous. Lead Nitrate And Sodium Hydroxide Formula.
From www.coursehero.com
[Solved] 13_ Aqueous lead (1]) nitrate, Ph[N03)2 undergoes a double Lead Nitrate And Sodium Hydroxide Formula The balanced equation will be calculated along. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Sodium chloride, sodium hydroxide, or. It consists of lead and nitrate ions and. Lead Nitrate And Sodium Hydroxide Formula.
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead Nitrate And Sodium Hydroxide Formula lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). enter an equation of an ionic chemical equation and press the balance button. First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. Sodium chloride, sodium hydroxide, or. it is a chemical compound. Lead Nitrate And Sodium Hydroxide Formula.
From protonstalk.com
Lead Nitrate Formula, Structure, Preparation & Properties ProtonsTalk Lead Nitrate And Sodium Hydroxide Formula which solution could be used to precipitate the barium ion, ba 2+, in a water sample: The balanced equation will be calculated along. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. enter an equation of an ionic chemical equation and press the balance button. . Lead Nitrate And Sodium Hydroxide Formula.
From www.slideserve.com
PPT Chemical Reactions and Equations PowerPoint Presentation, free Lead Nitrate And Sodium Hydroxide Formula it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. enter an equation of an ionic chemical equation and press the balance button. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. which solution could be used. Lead Nitrate And Sodium Hydroxide Formula.
From www.numerade.com
SOLVEDIf 96.3 mL of lead(II) nitrate solution reacts completely with Lead Nitrate And Sodium Hydroxide Formula Sodium chloride, sodium hydroxide, or. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it is a chemical compound with the formula pb (no₃)₂. enter an equation of an ionic chemical equation and press the balance button. It consists of lead and nitrate ions and appears as a colorless or. Lead Nitrate And Sodium Hydroxide Formula.
From www.slideshare.net
Preciptation reactions 1 Lead Nitrate And Sodium Hydroxide Formula it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. Sodium chloride, sodium hydroxide, or. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. it is a chemical compound with the formula pb (no₃)₂. lead(ii) ion reacts. Lead Nitrate And Sodium Hydroxide Formula.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead Nitrate And Sodium Hydroxide Formula it is a chemical compound with the formula pb (no₃)₂. The balanced equation will be calculated along. It consists of lead and nitrate ions and appears as a colorless or white. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: this page looks at the formation of some insoluble lead. Lead Nitrate And Sodium Hydroxide Formula.
From www.numerade.com
SOLVEDlead(Il) nitrate (aq) potassium iodide (a9) Observacion Lead Nitrate And Sodium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. Sodium chloride, sodium hydroxide, or. it is a chemical compound with the formula pb (no₃)₂. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. The balanced equation will be calculated along. It consists of lead. Lead Nitrate And Sodium Hydroxide Formula.
From bryan-chapter.blogspot.com
Copper Nitrate And Sodium Hydroxide Equation 33+ Pages Summary [800kb Lead Nitrate And Sodium Hydroxide Formula The balanced equation will be calculated along. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. enter an equation of an ionic chemical equation and press the balance button. it is a chemical compound with the formula pb (no₃)₂. which solution could be used to precipitate the. Lead Nitrate And Sodium Hydroxide Formula.
From brainly.in
Lead and nitrate in criss cross method Brainly.in Lead Nitrate And Sodium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. It consists of lead and nitrate ions and appears as a colorless or white. First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than. Lead Nitrate And Sodium Hydroxide Formula.
From www.slideserve.com
PPT 3. Solutions of sodium iodide and lead nitrate are mixed. I Lead Nitrate And Sodium Hydroxide Formula it is a chemical compound with the formula pb (no₃)₂. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. The balanced equation will be calculated along. First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. It consists of lead and nitrate ions. Lead Nitrate And Sodium Hydroxide Formula.
From exohqbjbg.blob.core.windows.net
Lead Hydroxide State Of Matter at Lester Brand blog Lead Nitrate And Sodium Hydroxide Formula which solution could be used to precipitate the barium ion, ba 2+, in a water sample: enter an equation of an ionic chemical equation and press the balance button. It consists of lead and nitrate ions and appears as a colorless or white. The balanced equation will be calculated along. Sodium chloride, sodium hydroxide, or. it is. Lead Nitrate And Sodium Hydroxide Formula.
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead Nitrate And Sodium Hydroxide Formula enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Sodium chloride, sodium hydroxide, or. First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: It. Lead Nitrate And Sodium Hydroxide Formula.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Nitrate And Sodium Hydroxide Formula It consists of lead and nitrate ions and appears as a colorless or white. Sodium chloride, sodium hydroxide, or. enter an equation of an ionic chemical equation and press the balance button. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: First, we need to write the molecular equation for the. Lead Nitrate And Sodium Hydroxide Formula.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Lead Nitrate And Sodium Hydroxide Formula this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. . Lead Nitrate And Sodium Hydroxide Formula.
From dxoezqcmu.blob.core.windows.net
Lead Hydroxide Ionic Formula at Clara Lott blog Lead Nitrate And Sodium Hydroxide Formula which solution could be used to precipitate the barium ion, ba 2+, in a water sample: enter an equation of an ionic chemical equation and press the balance button. it is a chemical compound with the formula pb (no₃)₂. The balanced equation will be calculated along. First, we need to write the molecular equation for the reaction. Lead Nitrate And Sodium Hydroxide Formula.
From www.youtube.com
How to Write the Net Ionic Equation for Pb(NO3)2 + (NH4)2CO3 = PbCO3 Lead Nitrate And Sodium Hydroxide Formula The balanced equation will be calculated along. Sodium chloride, sodium hydroxide, or. it is a chemical compound with the formula pb (no₃)₂. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii). Lead Nitrate And Sodium Hydroxide Formula.
From testbook.com
Lead (II) Nitrate Formula Structure, Preparation, & Uses Lead Nitrate And Sodium Hydroxide Formula it is a chemical compound with the formula pb (no₃)₂. enter an equation of an ionic chemical equation and press the balance button. It consists of lead and nitrate ions and appears as a colorless or white. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: this page looks. Lead Nitrate And Sodium Hydroxide Formula.
From www.slideshare.net
Precipitation react2 Lead Nitrate And Sodium Hydroxide Formula it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. enter an equation of an ionic chemical equation and press the balance button. lead(ii) ion reacts with aqueous ammonia to precipitate a white. Lead Nitrate And Sodium Hydroxide Formula.
From www.youtube.com
Equation for NaNO3 + H2O (Sodium nitrate + Water) YouTube Lead Nitrate And Sodium Hydroxide Formula Sodium chloride, sodium hydroxide, or. enter an equation of an ionic chemical equation and press the balance button. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: it is a chemical compound with the formula pb (no₃)₂. The balanced equation will be calculated along. It consists of lead and nitrate. Lead Nitrate And Sodium Hydroxide Formula.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium Lead Nitrate And Sodium Hydroxide Formula which solution could be used to precipitate the barium ion, ba 2+, in a water sample: First, we need to write the molecular equation for the reaction between lead (ii) nitrate and. The balanced equation will be calculated along. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation. Lead Nitrate And Sodium Hydroxide Formula.
From www.slideshare.net
Acids And Bases Lead Nitrate And Sodium Hydroxide Formula it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii). Sodium chloride, sodium hydroxide, or. this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii). Lead Nitrate And Sodium Hydroxide Formula.
From dxoojemzf.blob.core.windows.net
Sodium Hydroxide Lead Nitrate Balanced Equation at Leonard Griffin blog Lead Nitrate And Sodium Hydroxide Formula It consists of lead and nitrate ions and appears as a colorless or white. The balanced equation will be calculated along. which solution could be used to precipitate the barium ion, ba 2+, in a water sample: Sodium chloride, sodium hydroxide, or. it is a chemical compound with the formula pb (no₃)₂. First, we need to write the. Lead Nitrate And Sodium Hydroxide Formula.
From www.youtube.com
How to Write the Net Ionic Equation for HBr + NaOH = H2O + NaBr YouTube Lead Nitrate And Sodium Hydroxide Formula Sodium chloride, sodium hydroxide, or. it is a chemical compound with the formula pb (no₃)₂. it describes the formation of lead (ii) hydroxide, lead (ii) chloride, lead (ii) iodide and lead (ii) sulfate. enter an equation of an ionic chemical equation and press the balance button. lead(ii) ion reacts with aqueous ammonia to precipitate a white. Lead Nitrate And Sodium Hydroxide Formula.
From www.chegg.com
Solved EACTION 10A LEAD NITRATE (Pb(NO,), AND SODIUM Lead Nitrate And Sodium Hydroxide Formula this page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It consists of lead and nitrate ions and appears as a colorless or white. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. Sodium chloride, sodium hydroxide,. Lead Nitrate And Sodium Hydroxide Formula.