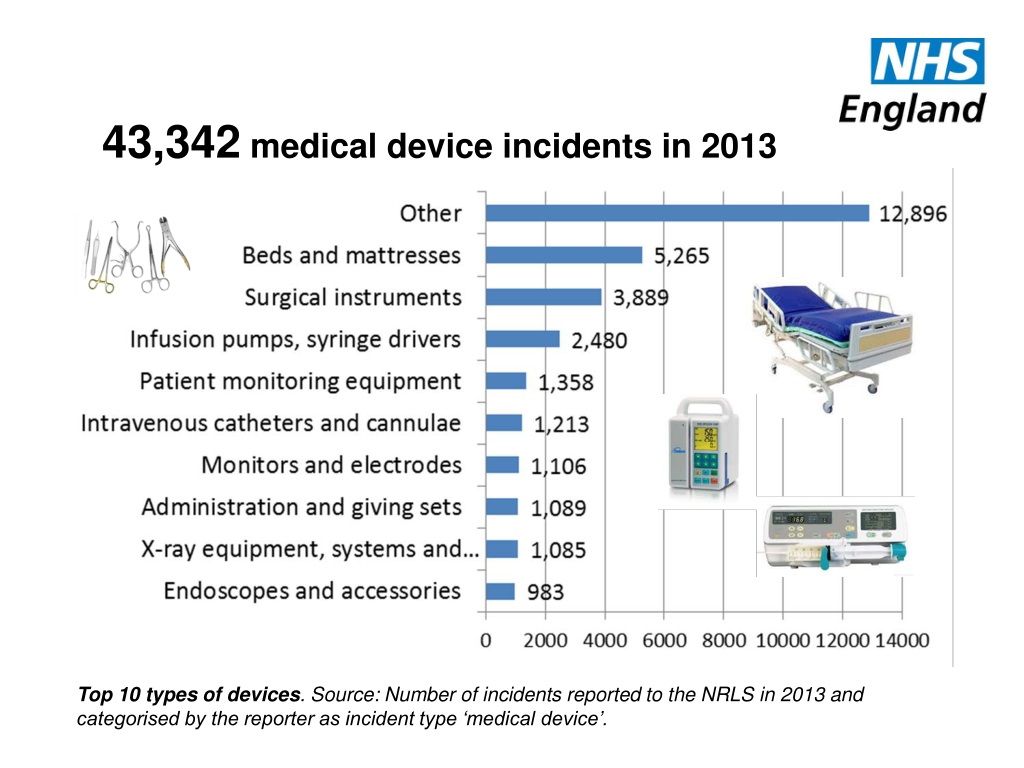
Medical Device Incident Definition . (1) ‘medical device’ means any. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. For the purposes of this regulation, the following definitions apply:
from www.slideserve.com
(1) ‘medical device’ means any. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. For the purposes of this regulation, the following definitions apply: the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for.
PPT National Medical Device Safety Network Better Together PowerPoint
Medical Device Incident Definition if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. (1) ‘medical device’ means any. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. For the purposes of this regulation, the following definitions apply: if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or.
From exowgjrmc.blob.core.windows.net
Mhra Medical Devices Incident Reporting at Leticia Ridley blog Medical Device Incident Definition the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. conducting successful medical device incident investigations is an essential aspect. Medical Device Incident Definition.
From www.scribd.com
Procedure04 Medical Device Incident PDF Medical Device Incident Definition An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. conducting successful medical device incident investigations is. Medical Device Incident Definition.
From medicaldevicehq.com
Medical device risk assessment The danger of division Part 3 Medical Device Incident Definition An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. (1) ‘medical device’ means any. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. Incident’ means. Medical Device Incident Definition.
From www.template.net
43+ Incident Report Formats PDF, Word, Pages Free & Premium Templates Medical Device Incident Definition conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. For the purposes of this regulation, the. Medical Device Incident Definition.
From www.orielstat.com
Medical Device Incident Reporting Timelines in 6 Major Markets Medical Device Incident Definition Incident’ means any malfunction or deterioration in the characteristics or performance of a device. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. (1) ‘medical device’ means any. For the. Medical Device Incident Definition.
From www.elexes.com
Ways to Report Medical Device Incidents in Australia via the MDIR Medical Device Incident Definition Incident’ means any malfunction or deterioration in the characteristics or performance of a device. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. For the purposes of this regulation, the. Medical Device Incident Definition.
From www.researchgate.net
(PDF) Correction to Amid COVID19 the importance of developing an Medical Device Incident Definition An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. (1) ‘medical device’ means any. if. Medical Device Incident Definition.
From www.youtube.com
What is a Patient (Medical) Incident Report Form? YouTube Medical Device Incident Definition the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. An incident. Medical Device Incident Definition.
From www.allbusinesstemplates.com
Medical Device Incident Investigation Report sample Templates at Medical Device Incident Definition For the purposes of this regulation, the following definitions apply: the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. if you are a manufacturer or importer, you must report deaths. Medical Device Incident Definition.
From cloudsecurityalliance.org
CSA Medical Device Incident Response Playbook CSA Medical Device Incident Definition conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any. . Medical Device Incident Definition.
From studylib.net
Submit a Medical Device Incident Report Medical Device Incident Definition (1) ‘medical device’ means any. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. For the purposes of this regulation, the following definitions apply: the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. . Medical Device Incident Definition.
From bcpslscentral.ca
Everything you need to know about medical device incident reporting for Medical Device Incident Definition the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. (1) ‘medical device’ means any. if you are a manufacturer. Medical Device Incident Definition.
From www.slideserve.com
PPT Incident Reporting PowerPoint Presentation, free download ID Medical Device Incident Definition (1) ‘medical device’ means any. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. Incident’. Medical Device Incident Definition.
From www.youtube.com
How to Manage Medical Device Incidents YouTube Medical Device Incident Definition An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. For the purposes of this regulation, the. Medical Device Incident Definition.
From www.slideserve.com
PPT Reporting System of Biocidal Vigilance PowerPoint Presentation Medical Device Incident Definition Incident’ means any malfunction or deterioration in the characteristics or performance of a device. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. For the purposes of. Medical Device Incident Definition.
From www.canada.ca
Guidance on releasing information from adverse reaction and medical Medical Device Incident Definition the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. (1) ‘medical device’ means any. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. For the purposes of this regulation, the following definitions apply: An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the. Medical Device Incident Definition.
From www.youtube.com
Types of Incident Incident/Accident/Near Miss/First Aid Case/Medical Medical Device Incident Definition For the purposes of this regulation, the following definitions apply: conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. (1) ‘medical device’ means any. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. the medical device reporting (mdr) regulation (21 cfr part 803) contains. Medical Device Incident Definition.
From www.slideserve.com
PPT National Medical Device Safety Network Better Together PowerPoint Medical Device Incident Definition Incident’ means any malfunction or deterioration in the characteristics or performance of a device. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. (1) ‘medical device’ means any. if you are a manufacturer or importer,. Medical Device Incident Definition.
From bcpslscentral.ca
Everything you need to know about medical device incident reporting for Medical Device Incident Definition Incident’ means any malfunction or deterioration in the characteristics or performance of a device. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory. Medical Device Incident Definition.
From circle.cloudsecurityalliance.org
CSA Medical Device Incident Response Playbook The Inner Circle Medical Device Incident Definition the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. if you are a manufacturer or. Medical Device Incident Definition.
From bcpslscentral.ca
Everything you need to know about medical device incident reporting for Medical Device Incident Definition the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. For the purposes of this regulation, the following definitions apply: if you are a manufacturer or importer, you must report deaths and serious injuries that. Medical Device Incident Definition.
From www.researchgate.net
(PDF) Medical Device Incident Reports by Professional Users in Finland Medical Device Incident Definition conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. For the purposes of this regulation, the following definitions apply: if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. the main difference between an “incident” and a “serious incident”. Medical Device Incident Definition.
From slideplayer.com
Protecting Canadians from Unsafe Drugs Act ppt download Medical Device Incident Definition if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. For the purposes of this regulation, the following definitions apply: An incident is simply defined by the regulation as a ‘malfunction or. Medical Device Incident Definition.
From www.pinterest.com
Medical Device Incident Report How to create a Medical Device Medical Device Incident Definition An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. For the purposes of this regulation, the following definitions apply: the main difference between an “incident” and a “serious incident” under the mdr is the severity of. Medical Device Incident Definition.
From www.researchgate.net
(PDF) Enhancing the effectiveness of medical device incident reporting Medical Device Incident Definition For the purposes of this regulation, the following definitions apply: if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. Incident’ means any malfunction or deterioration in the characteristics or performance. Medical Device Incident Definition.
From exowgjrmc.blob.core.windows.net
Mhra Medical Devices Incident Reporting at Leticia Ridley blog Medical Device Incident Definition the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. (1) ‘medical device’ means any. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. For the purposes of this regulation,. Medical Device Incident Definition.
From www.orielstat.com
Medical Device Incident Reporting Timelines in 6 Major Markets Medical Device Incident Definition For the purposes of this regulation, the following definitions apply: (1) ‘medical device’ means any. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. if you are a manufacturer or importer, you must report deaths. Medical Device Incident Definition.
From studylib.net
Medical device incident report form Medical Device Incident Definition Incident’ means any malfunction or deterioration in the characteristics or performance of a device. (1) ‘medical device’ means any. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the. Medical Device Incident Definition.
From microage.com
Understanding the 6 Elements of the Incident Response (IR) Process Medical Device Incident Definition (1) ‘medical device’ means any. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the. Medical Device Incident Definition.
From www.slideserve.com
PPT National Patient Safety Goals PowerPoint Presentation, free Medical Device Incident Definition if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. Incident’ means any malfunction or deterioration in the characteristics or performance of a device. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. An incident is simply defined by the. Medical Device Incident Definition.
From www.slideserve.com
PPT National Patient Safety Goals PowerPoint Presentation, free Medical Device Incident Definition (1) ‘medical device’ means any. For the purposes of this regulation, the following definitions apply: An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. Incident’ means any malfunction or deterioration in the characteristics or performance of. Medical Device Incident Definition.
From www.researchgate.net
(PDF) Electronic Health Records on the Top of Medical Device Incident Medical Device Incident Definition conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. the main difference between an “incident” and a “serious incident” under. Medical Device Incident Definition.
From www.performancehealthus.com
What is Incident Management? How to Prevent Harm in Healthcare Medical Device Incident Definition For the purposes of this regulation, the following definitions apply: An incident is simply defined by the regulation as a ‘malfunction or deterioration’ in the function or aesthetic. (1) ‘medical device’ means any. if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. Incident’ means any. Medical Device Incident Definition.
From www.slideserve.com
PPT Reporting System of Biocidal Vigilance PowerPoint Presentation Medical Device Incident Definition (1) ‘medical device’ means any. the medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for. conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the main difference between an “incident” and a “serious incident” under the mdr is the severity of the related. For the purposes of this. Medical Device Incident Definition.
From www.template.net
Medical Device Incident Report Template Edit Online & Download Medical Device Incident Definition if you are a manufacturer or importer, you must report deaths and serious injuries that your device has or may have caused or. For the purposes of this regulation, the following definitions apply: conducting successful medical device incident investigations is an essential aspect to achieving exceptionally safe,. the main difference between an “incident” and a “serious incident”. Medical Device Incident Definition.