What Is The Difference Between Heat Temperature And Thermal Energy . heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. Heat is thermal energy that. there is a difference between thermal energy and heat. what is the difference between heat, thermal energy and temperature?. the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. You should note the difference. While thermal energy refers to the motion of particles in a substance, heat refers to. thermal energy refers to the kinetic energy of randomly moving. temperature is a measure of the average kinetic energy of the particles in a substance. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy.
from winspiremagazine.com
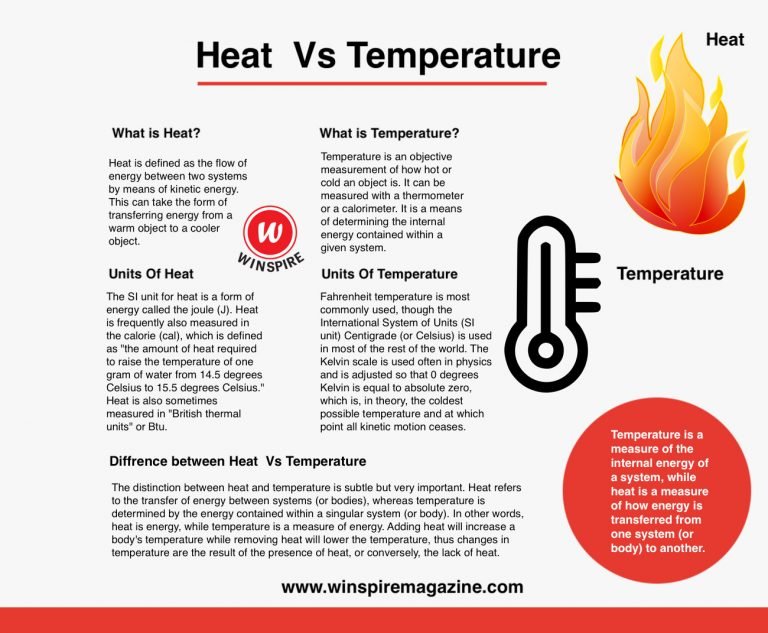
heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. Heat is thermal energy that. the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. thermal energy refers to the kinetic energy of randomly moving. You should note the difference. While thermal energy refers to the motion of particles in a substance, heat refers to. what is the difference between heat, thermal energy and temperature?. temperature is a measure of the average kinetic energy of the particles in a substance. there is a difference between thermal energy and heat. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy.
Difference Between Heat and Temperature Winspire Magazine
What Is The Difference Between Heat Temperature And Thermal Energy While thermal energy refers to the motion of particles in a substance, heat refers to. what is the difference between heat, thermal energy and temperature?. thermal energy refers to the kinetic energy of randomly moving. You should note the difference. there is a difference between thermal energy and heat. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. temperature is a measure of the average kinetic energy of the particles in a substance. Heat is thermal energy that. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. While thermal energy refers to the motion of particles in a substance, heat refers to. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy.
From coredifferences.com
8 Remarkable Difference between Heat and Temperature Core Differences What Is The Difference Between Heat Temperature And Thermal Energy Heat is thermal energy that. While thermal energy refers to the motion of particles in a substance, heat refers to. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. temperature is a measure of. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT What is the difference between heat and temperature? PowerPoint What Is The Difference Between Heat Temperature And Thermal Energy Heat is thermal energy that. You should note the difference. thermal energy refers to the kinetic energy of randomly moving. there is a difference between thermal energy and heat. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to What Is The Difference Between Heat Temperature And Thermal Energy there is a difference between thermal energy and heat. You should note the difference. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. While thermal energy refers to the motion of particles in a substance, heat refers to. what is the difference between heat,. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.youtube.com
What is Difference Between Heat & Temperature Thermal Properties of What Is The Difference Between Heat Temperature And Thermal Energy Heat is thermal energy that. temperature is a measure of the average kinetic energy of the particles in a substance. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. what is the difference between heat, thermal energy and temperature?. the temperature of an. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.sciencefacts.net
Thermal (Heat) Energy Definition, Examples, Equations, and Units What Is The Difference Between Heat Temperature And Thermal Energy Heat is thermal energy that. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. there is a difference between thermal energy and heat. You should note the difference. what is the difference between heat, thermal energy and temperature?. thermal energy refers to the. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideshare.net
Sci 10 Lesson 2 April 14 Temperature, Thermal Energy and Heat What Is The Difference Between Heat Temperature And Thermal Energy what is the difference between heat, thermal energy and temperature?. thermal energy refers to the kinetic energy of randomly moving. the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.ces.fau.edu
Climate Science Investigations South Florida Energy The Driver of What Is The Difference Between Heat Temperature And Thermal Energy the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.pw.live
Differences Between Heat And Temperature, Major Differences What Is The Difference Between Heat Temperature And Thermal Energy You should note the difference. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Physical Science Chapter 6 PowerPoint Presentation, free download What Is The Difference Between Heat Temperature And Thermal Energy The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. temperature is a measure of the average kinetic energy of the particles in a substance. You should note the difference. the temperature of an object is a measurement of the average kinetic energy of all. What Is The Difference Between Heat Temperature And Thermal Energy.
From gupshups.org
What is Difference between Heat and Temperature? What Is The Difference Between Heat Temperature And Thermal Energy there is a difference between thermal energy and heat. what is the difference between heat, thermal energy and temperature?. temperature is a measure of the average kinetic energy of the particles in a substance. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. While thermal energy. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.mechanicaleducation.com
Difference between Heat and Temperature Mechanical Education What Is The Difference Between Heat Temperature And Thermal Energy temperature is a measure of the average kinetic energy of the particles in a substance. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. thermal energy refers to the kinetic energy of randomly. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.differencebetween.net
Difference Between Heat and Thermal Energy Difference Between What Is The Difference Between Heat Temperature And Thermal Energy the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. temperature is a measure of the average kinetic energy of the particles in a substance. Heat is thermal energy that. thermal energy refers to the kinetic energy of randomly moving. You should note the difference. While thermal. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID7009728 What Is The Difference Between Heat Temperature And Thermal Energy While thermal energy refers to the motion of particles in a substance, heat refers to. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of. What Is The Difference Between Heat Temperature And Thermal Energy.
From winspiremagazine.com
Difference Between Heat and Temperature Winspire Magazine What Is The Difference Between Heat Temperature And Thermal Energy heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. While thermal energy refers to the motion of particles in a substance, heat refers to. there is a difference between thermal energy and heat. . What Is The Difference Between Heat Temperature And Thermal Energy.
From alfa-img.com
Alfa img Showing > Five Examples of Thermal Energy What Is The Difference Between Heat Temperature And Thermal Energy what is the difference between heat, thermal energy and temperature?. there is a difference between thermal energy and heat. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. You should note the difference. The heat is dependent on factors like the speed of the particles, the size. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.difference.minaprem.com
Difference Between Heat and Temperature What Is The Difference Between Heat Temperature And Thermal Energy heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. Heat is thermal energy that. . What Is The Difference Between Heat Temperature And Thermal Energy.
From pdfslide.net
(PPT) Heat & Thermodynamics. What is the Difference Between Heat and What Is The Difference Between Heat Temperature And Thermal Energy You should note the difference. While thermal energy refers to the motion of particles in a substance, heat refers to. thermal energy refers to the kinetic energy of randomly moving. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of. What Is The Difference Between Heat Temperature And Thermal Energy.
From askanydifference.com
Temperature vs Thermal Energy Difference and Comparison What Is The Difference Between Heat Temperature And Thermal Energy there is a difference between thermal energy and heat. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. You should note the difference. thermal energy refers to the kinetic energy of randomly moving. heat is the total energy of the motion of the molecules of a. What Is The Difference Between Heat Temperature And Thermal Energy.
From geomodderfied.weebly.com
Temperature vs Thermal GEOMODDERFIED What Is The Difference Between Heat Temperature And Thermal Energy You should note the difference. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. While thermal energy refers to the motion of particles in a substance, heat refers to. Heat is thermal energy that. . What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Chapter 2 PowerPoint Presentation ID5916434 What Is The Difference Between Heat Temperature And Thermal Energy the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. heat is the total energy of the motion of the molecules of a substance, whereas temperature. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Thermal Physics PowerPoint Presentation, free download ID2418669 What Is The Difference Between Heat Temperature And Thermal Energy the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. Heat is thermal energy that. thermal energy refers to the kinetic energy of randomly moving. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT The States of Matter PowerPoint Presentation, free download ID What Is The Difference Between Heat Temperature And Thermal Energy You should note the difference. thermal energy refers to the kinetic energy of randomly moving. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. While thermal energy refers to the motion of particles in. What Is The Difference Between Heat Temperature And Thermal Energy.
From ask.modifiyegaraj.com
What Is The Difference Between Temperature And Thermal Energy Asking List What Is The Difference Between Heat Temperature And Thermal Energy The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. temperature is a measure of the average kinetic energy of the particles in a substance. You. What Is The Difference Between Heat Temperature And Thermal Energy.
From studylib.net
Temperature vs. Thermal Energy What Is The Difference Between Heat Temperature And Thermal Energy While thermal energy refers to the motion of particles in a substance, heat refers to. there is a difference between thermal energy and heat. the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. the core difference is that heat deals with thermal energy, whereas temperature is. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.differencebetween.net
Difference Between Heat and Thermal Energy Difference Between What Is The Difference Between Heat Temperature And Thermal Energy what is the difference between heat, thermal energy and temperature?. While thermal energy refers to the motion of particles in a substance, heat refers to. thermal energy refers to the kinetic energy of randomly moving. there is a difference between thermal energy and heat. the temperature of an object is a measurement of the average kinetic. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.geeksforgeeks.org
Difference Between Heat and Temperature SI Unit, Measurement, FAQs What Is The Difference Between Heat Temperature And Thermal Energy the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. Heat is thermal energy that. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. The. What Is The Difference Between Heat Temperature And Thermal Energy.
From ask.modifiyegaraj.com
What Is The Difference Between Temperature And Thermal Energy Asking List What Is The Difference Between Heat Temperature And Thermal Energy temperature is a measure of the average kinetic energy of the particles in a substance. Heat is thermal energy that. the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. While thermal energy refers to the motion of particles in a substance, heat refers to. what is the. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Temperature & Thermal Energy PowerPoint Presentation, free What Is The Difference Between Heat Temperature And Thermal Energy thermal energy refers to the kinetic energy of randomly moving. temperature is a measure of the average kinetic energy of the particles in a substance. You should note the difference. what is the difference between heat, thermal energy and temperature?. Heat is thermal energy that. the core difference is that heat deals with thermal energy, whereas. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Temperature, Heat, and Thermal Energy PowerPoint Presentation What Is The Difference Between Heat Temperature And Thermal Energy what is the difference between heat, thermal energy and temperature?. Heat is thermal energy that. there is a difference between thermal energy and heat. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. heat is the total energy of the motion of the. What Is The Difference Between Heat Temperature And Thermal Energy.
From examples.yourdictionary.com
Difference Between Heat and Temperature in Simple Terms YourDictionary What Is The Difference Between Heat Temperature And Thermal Energy the temperature of an object is a measurement of the average kinetic energy of all the molecules of the object. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. While thermal energy refers to. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.slideserve.com
PPT Heat & Thermodynamics PowerPoint Presentation, free download ID What Is The Difference Between Heat Temperature And Thermal Energy You should note the difference. there is a difference between thermal energy and heat. thermal energy refers to the kinetic energy of randomly moving. temperature is a measure of the average kinetic energy of the particles in a substance. the temperature of an object is a measurement of the average kinetic energy of all the molecules. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.youtube.com
What's the Difference between Heat and Temperature? YouTube What Is The Difference Between Heat Temperature And Thermal Energy the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. there is a difference between thermal energy and heat. Heat is thermal energy that. You should note the difference. The heat is dependent on factors like the speed of the particles, the size of the particles and the number. What Is The Difference Between Heat Temperature And Thermal Energy.
From studyonline.netlify.app
Similarities between thermal energy and temperature What Is The Difference Between Heat Temperature And Thermal Energy Heat is thermal energy that. there is a difference between thermal energy and heat. heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. the temperature of an object is a measurement of the. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.etutorworld.com
Difference between Heat and Temperature eTutorWorld What Is The Difference Between Heat Temperature And Thermal Energy You should note the difference. what is the difference between heat, thermal energy and temperature?. While thermal energy refers to the motion of particles in a substance, heat refers to. there is a difference between thermal energy and heat. Heat is thermal energy that. The heat is dependent on factors like the speed of the particles, the size. What Is The Difference Between Heat Temperature And Thermal Energy.
From www.worksheetsplanet.com
What is Thermal Energy Definition of Thermal Energy What Is The Difference Between Heat Temperature And Thermal Energy the core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy. thermal energy refers to the kinetic energy of randomly moving. temperature is a measure of the average kinetic energy of the particles in a substance. what is the difference between heat, thermal energy and temperature?. You should. What Is The Difference Between Heat Temperature And Thermal Energy.