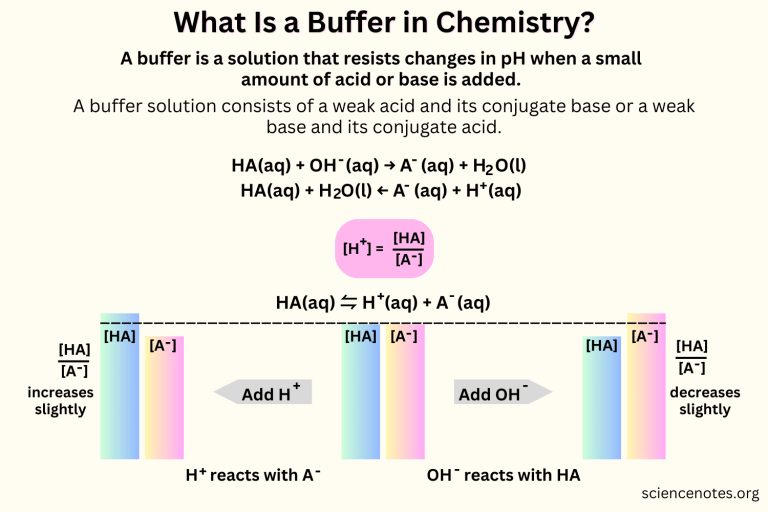What Are Buffer Solutions Used For A Level Biology . Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are important in biological systems because of their ability to maintain. Revision notes on 2.4.3 enzyme activity: Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biochemical experiments routinely require a buffer. Protein buffer systems work predominantly inside cells. In this laboratory you will cover the basics of buffer preparation and test the buffering. Most commonly, the buffer solution.
from sciencenotes.org
Protein buffer systems work predominantly inside cells. Buffers are important in biological systems because of their ability to maintain. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the buffering. Most commonly, the buffer solution. Revision notes on 2.4.3 enzyme activity:
Buffer Definition and Examples in Chemistry
What Are Buffer Solutions Used For A Level Biology Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Biochemical experiments routinely require a buffer. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. In this laboratory you will cover the basics of buffer preparation and test the buffering. Buffers are important in biological systems because of their ability to maintain. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Protein buffer systems work predominantly inside cells. Revision notes on 2.4.3 enzyme activity: Most commonly, the buffer solution. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In this laboratory you will cover the basics of buffer preparation and test the buffering. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Buffers are important in biological systems because of their ability. What Are Buffer Solutions Used For A Level Biology.
From www.toppr.com
Buffer Solutions Definition, Types, Preparation, Examples and Videos What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the buffering. Revision notes on 2.4.3 enzyme activity: Most commonly, the buffer solution. The buffer systems functioning in blood plasma include plasma proteins,. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Are Buffer Solutions Used For A Level Biology Most commonly, the buffer solution. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Protein buffer systems work predominantly inside cells. Biochemical experiments routinely require a buffer. In this laboratory you will cover the. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint What Are Buffer Solutions Used For A Level Biology Most commonly, the buffer solution. Protein buffer systems work predominantly inside cells. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their ability to maintain. A buffer solution is one that resists a change in ph on the addition of acid (h+) or. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Are Buffer Solutions Used For A Level Biology A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Revision notes on 2.4.3 enzyme activity: Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In this laboratory you will cover the basics. What Are Buffer Solutions Used For A Level Biology.
From www.pinterest.com
Buffer Buffer solution, Easy science, Flashcards What Are Buffer Solutions Used For A Level Biology Biochemical experiments routinely require a buffer. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more. What Are Buffer Solutions Used For A Level Biology.
From vocal.media
Preparation of Buffers Solution FYI What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Ph for the ocr a level biology syllabus, written by the biology experts at. What Are Buffer Solutions Used For A Level Biology.
From oda-dink.blogspot.com
What Is Buffer Solution Solved Suppose A Buffer Solution Is Made What Are Buffer Solutions Used For A Level Biology A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Revision notes on 2.4.3 enzyme activity: Buffers are important in biological systems because of their ability to maintain. Most commonly, the buffer solution. Buffers are solutions that moderate ph changes when an. What Are Buffer Solutions Used For A Level Biology.
From loeukppln.blob.core.windows.net
Buffers Ap Biology at Venessa Baird blog What Are Buffer Solutions Used For A Level Biology The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Most commonly, the buffer solution. Buffers are important in biological systems because of their ability to maintain. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one that resists a change in ph. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT BUFFER SOLUTIONS A guide for A level students PowerPoint What Are Buffer Solutions Used For A Level Biology In this laboratory you will cover the basics of buffer preparation and test the buffering. Revision notes on 2.4.3 enzyme activity: Most commonly, the buffer solution. Protein buffer systems work predominantly inside cells. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer solution is one that resists a change in. What Are Buffer Solutions Used For A Level Biology.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy What Are Buffer Solutions Used For A Level Biology Protein buffer systems work predominantly inside cells. In this laboratory you will cover the basics of buffer preparation and test the buffering. Revision notes on 2.4.3 enzyme activity: The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are important in biological systems because of their ability to maintain. Buffers are solutions. What Are Buffer Solutions Used For A Level Biology.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution What Are Buffer Solutions Used For A Level Biology Most commonly, the buffer solution. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. In this laboratory you will cover the basics of buffer preparation and test the buffering. Protein buffer systems work predominantly. What Are Buffer Solutions Used For A Level Biology.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Are Buffer Solutions Used For A Level Biology Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Most commonly, the buffer solution. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−),. What Are Buffer Solutions Used For A Level Biology.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download What Are Buffer Solutions Used For A Level Biology Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Protein buffer systems work predominantly inside cells. Buffers are important in biological systems because of their ability to maintain. Biochemical experiments routinely require a buffer. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system.. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Revision notes on 2.4.3 enzyme activity: Most commonly, the buffer solution. Protein buffer systems work predominantly inside cells. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one that resists a change. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Are Buffer Solutions Used For A Level Biology Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water.. What Are Buffer Solutions Used For A Level Biology.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Are Buffer Solutions Used For A Level Biology Most commonly, the buffer solution. In this laboratory you will cover the basics of buffer preparation and test the buffering. Protein buffer systems work predominantly inside cells. Biochemical experiments routinely require a buffer. Revision notes on 2.4.3 enzyme activity: The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are important in. What Are Buffer Solutions Used For A Level Biology.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Are Buffer Solutions Used For A Level Biology Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Buffers are important in biological systems because of their ability to maintain. Revision notes on 2.4.3 enzyme activity: A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Most commonly, the buffer solution. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Biochemical experiments routinely require a buffer. Protein buffer systems. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Ph for the ocr a level biology syllabus, written by the biology experts at. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID What Are Buffer Solutions Used For A Level Biology The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this laboratory you will cover the basics of buffer preparation and test the buffering. Protein buffer systems work predominantly inside cells. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more. What Are Buffer Solutions Used For A Level Biology.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Are Buffer Solutions Used For A Level Biology A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Buffers are important in biological systems because of their ability to maintain. Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the. What Are Buffer Solutions Used For A Level Biology.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Are Buffer Solutions Used For A Level Biology Biochemical experiments routinely require a buffer. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Revision notes on 2.4.3 enzyme activity: Buffers are solutions. What Are Buffer Solutions Used For A Level Biology.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In this laboratory you will cover the basics of buffer preparation and test the buffering. Buffers are important in biological systems because of their ability to maintain. Revision notes on 2.4.3 enzyme activity: Ph for the ocr a level biology syllabus, written. What Are Buffer Solutions Used For A Level Biology.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers What Are Buffer Solutions Used For A Level Biology In this laboratory you will cover the basics of buffer preparation and test the buffering. Most commonly, the buffer solution. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers are important in biological systems. What Are Buffer Solutions Used For A Level Biology.
From www.youtube.com
Calculating pH of buffer solution with Strong Acid YouTube What Are Buffer Solutions Used For A Level Biology Revision notes on 2.4.3 enzyme activity: Protein buffer systems work predominantly inside cells. Biochemical experiments routinely require a buffer. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Most commonly, the buffer solution. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. The. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT Buffers and its uses. PowerPoint Presentation, free download ID What Are Buffer Solutions Used For A Level Biology Buffers are important in biological systems because of their ability to maintain. In this laboratory you will cover the basics of buffer preparation and test the buffering. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Protein buffer systems work predominantly inside cells. Most commonly, the buffer solution. A buffer solution is. What Are Buffer Solutions Used For A Level Biology.
From peacecommission.kdsg.gov.ng
Buffer Solutions Definition, Types, Mechanisms, Uses Embibe What Are Buffer Solutions Used For A Level Biology Biochemical experiments routinely require a buffer. Most commonly, the buffer solution. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Revision notes on 2.4.3 enzyme activity: Protein buffer systems work predominantly inside cells. Ph for the ocr a level biology syllabus,. What Are Buffer Solutions Used For A Level Biology.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Are Buffer Solutions Used For A Level Biology Revision notes on 2.4.3 enzyme activity: Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Protein buffer systems work predominantly inside cells. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biochemical experiments routinely require a buffer. A buffer solution is one that resists. What Are Buffer Solutions Used For A Level Biology.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint What Are Buffer Solutions Used For A Level Biology The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume. What Are Buffer Solutions Used For A Level Biology.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid What Are Buffer Solutions Used For A Level Biology Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Buffers are important in biological systems because of their ability to maintain. Protein buffer systems work predominantly inside cells. Biochemical experiments routinely require a buffer. Revision notes on 2.4.3 enzyme activity: Most commonly, the buffer solution. Buffers are solutions that moderate ph changes. What Are Buffer Solutions Used For A Level Biology.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Are Buffer Solutions Used For A Level Biology Protein buffer systems work predominantly inside cells. Biochemical experiments routinely require a buffer. Most commonly, the buffer solution. Buffers are important in biological systems because of their ability to maintain. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one that resists a change in ph on the addition of. What Are Buffer Solutions Used For A Level Biology.
From studylib.net
Buffer Solutions What Are Buffer Solutions Used For A Level Biology Biochemical experiments routinely require a buffer. Buffers are important in biological systems because of their ability to maintain. A buffer solution is one that resists a change in ph on the addition of acid (h+) or base (oh−), more effectively than an equal volume of water. Protein buffer systems work predominantly inside cells. Revision notes on 2.4.3 enzyme activity: Buffers. What Are Buffer Solutions Used For A Level Biology.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biochemical experiments routinely require a buffer. Ph for the ocr a level biology syllabus, written by the biology experts at save my exams. Buffers are important in biological systems because of their ability to maintain. Revision notes on 2.4.3 enzyme activity: Most. What Are Buffer Solutions Used For A Level Biology.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies What Are Buffer Solutions Used For A Level Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biochemical experiments routinely require a buffer. Protein buffer systems work predominantly inside cells. In this laboratory you will cover the basics of buffer preparation and test the buffering. A buffer solution is one that resists a change in ph on the addition. What Are Buffer Solutions Used For A Level Biology.