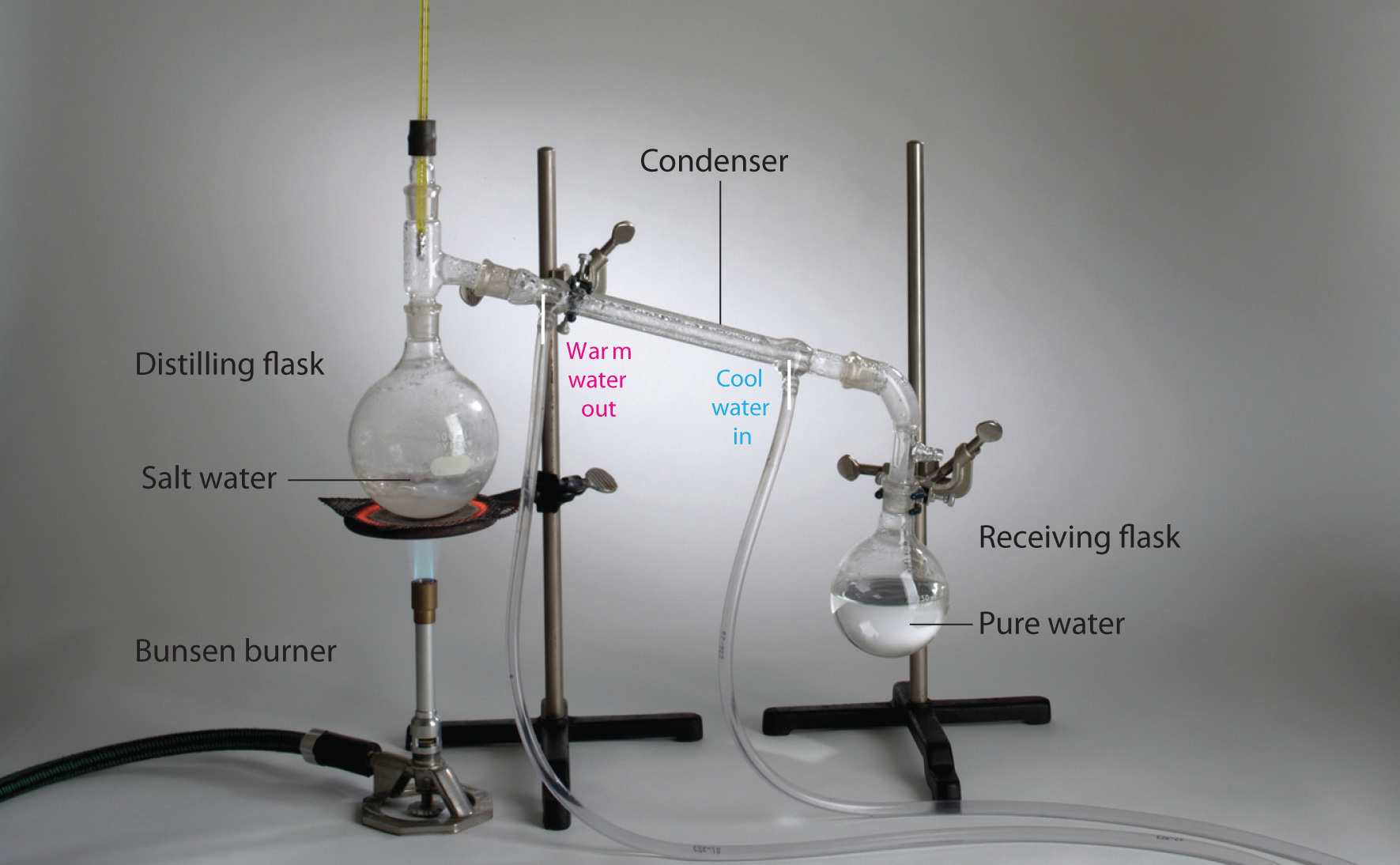
Filtration And Distillation . Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is a purification process where the components. Distillation (as discussed in analysis: Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Includes kit list and safety instructions.
from chem.libretexts.org
Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation is a purification process where the components. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Includes kit list and safety instructions. Distillation (as discussed in analysis:
1A.3 Classifying Matter Chemistry LibreTexts
Filtration And Distillation There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Includes kit list and safety instructions. Distillation (as discussed in analysis: Distillation is a purification process where the components. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar.
From speichim.com
Main principes of distillation Speichim Processing Valls Química Filtration And Distillation Includes kit list and safety instructions. Distillation is a purification process where the components. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation (as discussed in analysis: Distillation. Filtration And Distillation.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Filtration And Distillation In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Distillation is an effective. Filtration And Distillation.
From video.yckmc.edu.hk
Chemistry tutorialCh621Filtration, crystallization, simple distillation and fractional Filtration And Distillation Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation (as discussed in analysis: Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass.. Filtration And Distillation.
From www.vecteezy.com
Diagram showing Distillation Separating Mixtures 2896364 Vector Art at Vecteezy Filtration And Distillation Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation is a purification process where the components. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that. Filtration And Distillation.
From www.studypage.in
Separation of Mixtures Study Page Filtration And Distillation Distillation (as discussed in analysis: Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is a purification process where the components. Distillation can be used to purify water, make alcohols, or separate. Filtration And Distillation.
From mavink.com
Distillation Phase Diagram Filtration And Distillation Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation. Filtration And Distillation.
From www.mindomo.com
How can I demonstrate an understanding of Mind Map Filtration And Distillation Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the. Filtration And Distillation.
From www.slideserve.com
PPT Simple Distillation PowerPoint Presentation, free download ID2803725 Filtration And Distillation Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation (as discussed in analysis: There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation can be. Filtration And Distillation.
From mavink.com
Labelled Diagram Of Distillation Filtration And Distillation In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Includes kit list and safety instructions. Distillation. Filtration And Distillation.
From www.researchgate.net
Schematic diagram of the membrane distillation setup Download Scientific Diagram Filtration And Distillation Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Includes kit list and safety instructions. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of. Filtration And Distillation.
From www.geeksforgeeks.org
Separation by Distillation Filtration And Distillation Distillation is a purification process where the components. Distillation (as discussed in analysis: Includes kit list and safety instructions. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation can be used to purify. Filtration And Distillation.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Filtration And Distillation Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation is a purification process where the components. Distillation (as discussed in analysis: Try this class experiment to practise. Filtration And Distillation.
From 12014dorcascharityng.weebly.com
FILTRATION,EVAPORATION,DISTILLATION AND PAPER CHROMATOGRAPHY 3E1 Dorcas (4) Filtration And Distillation In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Distillation is an effective. Filtration And Distillation.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Filtration And Distillation Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Includes kit list and safety instructions. Distillation is a purification process where the components. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation is the use. Filtration And Distillation.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Filtration And Distillation Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation is a purification process where the components. Distillation (as discussed in analysis: Includes kit list and safety instructions. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and. Filtration And Distillation.
From www.yaclass.in
Simple distillation — lesson. Science CBSE, Class 9. Filtration And Distillation In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Includes kit list and. Filtration And Distillation.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Filtration And Distillation Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Try this class experiment to practise manipulating. Filtration And Distillation.
From www.aquaportail.com
Distillation définition et explications AquaPortail Filtration And Distillation Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation. Filtration And Distillation.
From yairfersgordon.blogspot.com
How to Separate Liquids With Different Boiling Points Filtration And Distillation Distillation is a purification process where the components. Includes kit list and safety instructions. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Try this class experiment to practise manipulating mixtures of soluble and. Filtration And Distillation.
From microbenotes.com
Water Distiller Principle, Parts, Types, Uses, Examples Filtration And Distillation Includes kit list and safety instructions. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation is an effective method to separate mixtures that are comprised of. Filtration And Distillation.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Filtration And Distillation Includes kit list and safety instructions. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids.. Filtration And Distillation.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Filtration And Distillation In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation is a purification process where the components. Distillation can be used to purify water, make alcohols, or separate petroleum into various components. Filtration And Distillation.
From www.waterpronto.com
Water Purification By Distillation Process Explained Filtration And Distillation Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration. Filtration And Distillation.
From www.thoughtco.com
What Is Distillation? Principles and Uses Filtration And Distillation Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation (as discussed in analysis: Distillation is the use of heat to separate the components of a liquid. Filtration And Distillation.
From help.chemix.org
Drawing distillation setups Chemix Help Center Filtration And Distillation Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation is a purification process where the components. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration. Filtration And Distillation.
From minisite.proj.hkedcity.net
Separation and Purification Methods Filtration And Distillation Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation (as discussed in analysis: In the simplest terms, a distillation involves boiling a liquid, then condensing the gas. Filtration And Distillation.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Filtration And Distillation Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. In. Filtration And Distillation.
From en.wikipedia.org
Distillation Wikipedia Filtration And Distillation Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the. Filtration And Distillation.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Filtration And Distillation Includes kit list and safety instructions. Distillation (as discussed in analysis: There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that. Filtration And Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry LibreTexts Filtration And Distillation There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is a purification process where the components. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline to tar. Try this class experiment to practise manipulating mixtures of soluble and insoluble. Filtration And Distillation.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Filtration And Distillation Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation (as discussed in analysis: Distillation is a purification process where the components. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or. Filtration And Distillation.
From www.researchgate.net
Distillation for Water Purification Download Scientific Diagram Filtration And Distillation Distillation is a purification process where the components. Distillation is the use of heat to separate the components of a liquid and/or gas, while filtration is the separation of solids from a fluid (either a gas or a liquid) by allowing the fluid to pass. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating. Filtration And Distillation.
From www.vedantu.com
What do you mean by filtration. Explain with a diagram. Filtration And Distillation There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Try this class experiment to practise manipulating mixtures of soluble and insoluble materials by separating sand and salt. Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. Distillation is the use of heat to separate the. Filtration And Distillation.
From www.bbc.co.uk
Distillation BBC Bitesize Filtration And Distillation Distillation (as discussed in analysis: Distillation is an effective method to separate mixtures that are comprised of two or more pure liquids. There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation can be used to purify water, make alcohols, or separate petroleum into various components that range from natural gas to gasoline. Filtration And Distillation.
From www.labrotovap.com
What are the different types of distillation process? Lab Instrument Manufacturer Filtration And Distillation In the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Distillation is a purification process where the components. Distillation (as discussed in analysis: There are different ways to separate mixtures, such as filtration, crystallisation, simple distillation, fractional distillation and. Distillation is an effective method to separate mixtures that are comprised of. Filtration And Distillation.