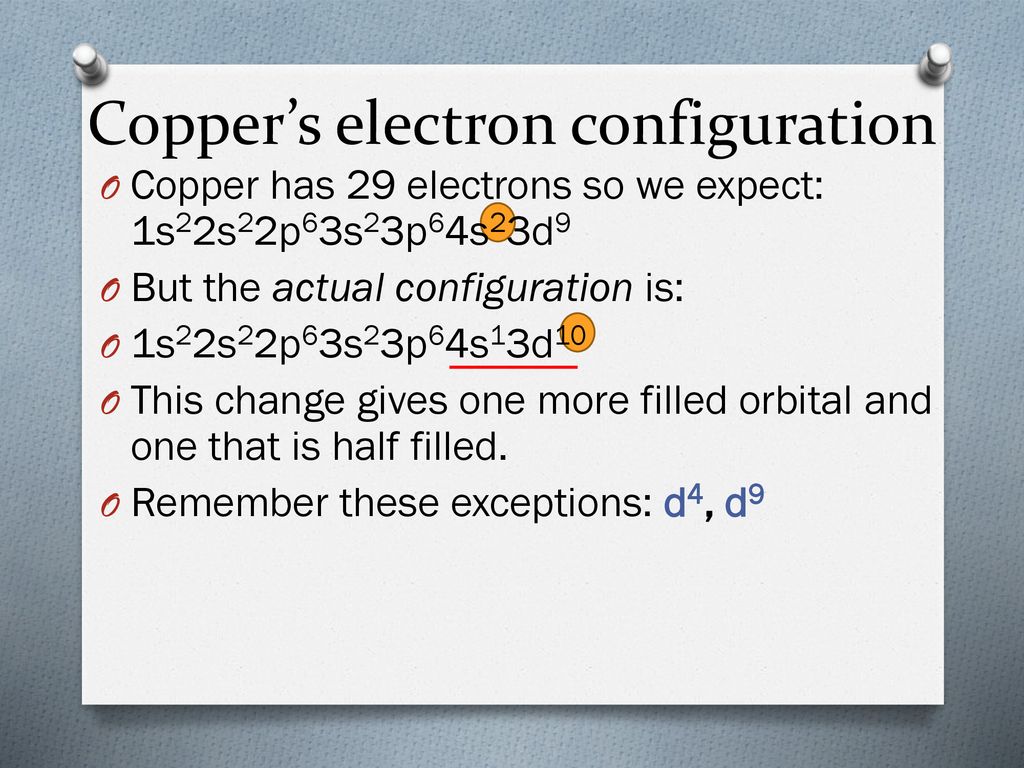
Copper Exception Electron Configuration . Cu = [ar] 4s1 3d10. exceptions to expected electron configurations. When we write the configuration we'll put all 29 electrons in. Cr = [ar] 4s1 3d5. To understand why this occurs,. once we have the configuration for cu, the ions are simple. this chemistry video tutorial covers exceptions in electron configuration. the actual electron configurations are: Similar to chromium, this exception occurs because having a. Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. There are some exceptions to the order of filling of orbitals that is shown in figure. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. The actual electron configuration may be rationalized in.
from slideplayer.com
When we write the configuration we'll put all 29 electrons in. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. once we have the configuration for cu, the ions are simple. To understand why this occurs,. Cu = [ar] 4s1 3d10. The actual electron configuration may be rationalized in. Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. the actual electron configurations are: Cr = [ar] 4s1 3d5.
Light Energy and Electron Configurations ppt download
Copper Exception Electron Configuration copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. Similar to chromium, this exception occurs because having a. When we write the configuration we'll put all 29 electrons in. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. this chemistry video tutorial covers exceptions in electron configuration. the actual electron configurations are: similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. Cu = [ar] 4s1 3d10. exceptions to expected electron configurations. To understand why this occurs,. The actual electron configuration may be rationalized in. once we have the configuration for cu, the ions are simple. Cr = [ar] 4s1 3d5. There are some exceptions to the order of filling of orbitals that is shown in figure.
From slideplayer.com
Electronic Structure of Atoms ppt download Copper Exception Electron Configuration The actual electron configuration may be rationalized in. When we write the configuration we'll put all 29 electrons in. this chemistry video tutorial covers exceptions in electron configuration. There are some exceptions to the order of filling of orbitals that is shown in figure. To understand why this occurs,. Cu = [ar] 4s1 3d10. Similar to chromium, this exception. Copper Exception Electron Configuration.
From slideplayer.com
Atomic Structure. ppt download Copper Exception Electron Configuration Similar to chromium, this exception occurs because having a. The actual electron configuration may be rationalized in. Cr = [ar] 4s1 3d5. There are some exceptions to the order of filling of orbitals that is shown in figure. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. Revise chromium and. Copper Exception Electron Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper Exception Electron Configuration The actual electron configuration may be rationalized in. To understand why this occurs,. Cu = [ar] 4s1 3d10. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. When we write the configuration we'll put all 29 electrons in. this chemistry video tutorial covers exceptions in electron configuration.. Copper Exception Electron Configuration.
From www.slideserve.com
PPT Electron Configuration of Ions PowerPoint Presentation, free Copper Exception Electron Configuration the actual electron configurations are: Cu = [ar] 4s1 3d10. Similar to chromium, this exception occurs because having a. exceptions to expected electron configurations. To understand why this occurs,. There are some exceptions to the order of filling of orbitals that is shown in figure. once we have the configuration for cu, the ions are simple. . Copper Exception Electron Configuration.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Exception Electron Configuration once we have the configuration for cu, the ions are simple. There are some exceptions to the order of filling of orbitals that is shown in figure. Similar to chromium, this exception occurs because having a. When we write the configuration we'll put all 29 electrons in. exceptions to expected electron configurations. The actual electron configuration may be. Copper Exception Electron Configuration.
From www.slideserve.com
PPT Chapter 5 “Electrons in Atoms” PowerPoint Presentation, free Copper Exception Electron Configuration copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. Cr = [ar] 4s1 3d5. When we write the configuration we'll put all 29 electrons in. the actual electron configurations are: Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. Cu = [ar]. Copper Exception Electron Configuration.
From gionopvkx.blob.core.windows.net
Copper Electron Configuration Class 10 at Ivan Peterson blog Copper Exception Electron Configuration Cr = [ar] 4s1 3d5. this chemistry video tutorial covers exceptions in electron configuration. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. exceptions to expected electron configurations. To understand why this occurs,. once we have the configuration for cu, the ions are simple. . Copper Exception Electron Configuration.
From slideplayer.com
Light Energy and Electron Configurations ppt download Copper Exception Electron Configuration Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. once we have the configuration for cu, the ions are simple. There are some exceptions to the order of filling of orbitals that is shown in figure. the actual electron configurations are: exceptions to expected electron configurations. since the core electron shells. Copper Exception Electron Configuration.
From www.youtube.com
Exceptions to Aufbau principle Chromium and Copper configuration BSc Copper Exception Electron Configuration exceptions to expected electron configurations. The actual electron configuration may be rationalized in. To understand why this occurs,. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. once we have the configuration for cu, the ions are simple. the actual electron configurations are: Similar to. Copper Exception Electron Configuration.
From slideplayer.com
Electron Configuration ppt video online download Copper Exception Electron Configuration similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. exceptions to expected electron configurations. Cr = [ar] 4s1 3d5. Similar to chromium, this exception occurs. Copper Exception Electron Configuration.
From www.youtube.com
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Copper Ions Copper Exception Electron Configuration since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. The actual electron configuration may be rationalized in. Cu = [ar] 4s1 3d10. Similar to. Copper Exception Electron Configuration.
From slideplayer.com
Bohr’s Model of the Atom ppt download Copper Exception Electron Configuration There are some exceptions to the order of filling of orbitals that is shown in figure. The actual electron configuration may be rationalized in. the actual electron configurations are: Cu = [ar] 4s1 3d10. Similar to chromium, this exception occurs because having a. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s. Copper Exception Electron Configuration.
From slideplayer.com
Electron Configurations ppt download Copper Exception Electron Configuration Cr = [ar] 4s1 3d5. exceptions to expected electron configurations. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. Cu = [ar] 4s1 3d10. To understand why this occurs,. this chemistry video tutorial covers exceptions in electron configuration. copper has an electron configuration of [ar] 3d 10. Copper Exception Electron Configuration.
From slideplayer.com
Chapter 5 “Electrons in Atoms” ppt download Copper Exception Electron Configuration When we write the configuration we'll put all 29 electrons in. this chemistry video tutorial covers exceptions in electron configuration. To understand why this occurs,. Cu = [ar] 4s1 3d10. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. copper has an electron. Copper Exception Electron Configuration.
From slideplayer.com
Exceptions to Predicted Electron Configurations ppt download Copper Exception Electron Configuration Similar to chromium, this exception occurs because having a. this chemistry video tutorial covers exceptions in electron configuration. Cu = [ar] 4s1 3d10. once we have the configuration for cu, the ions are simple. To understand why this occurs,. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9. Copper Exception Electron Configuration.
From slideplayer.com
Electronic Structure of the Atom ppt download Copper Exception Electron Configuration this chemistry video tutorial covers exceptions in electron configuration. There are some exceptions to the order of filling of orbitals that is shown in figure. Cr = [ar] 4s1 3d5. The actual electron configuration may be rationalized in. once we have the configuration for cu, the ions are simple. Revise chromium and copper's unique electron configurations as examples. Copper Exception Electron Configuration.
From www.youtube.com
Electronic configuration of chromium and copper called exceptions Copper Exception Electron Configuration Cu = [ar] 4s1 3d10. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. There are some exceptions to the order of filling of orbitals that is shown in figure. exceptions to expected electron configurations. Similar to chromium, this exception occurs because having a.. Copper Exception Electron Configuration.
From slideplayer.com
Hang posters. Then review video ppt download Copper Exception Electron Configuration exceptions to expected electron configurations. Similar to chromium, this exception occurs because having a. the actual electron configurations are: When we write the configuration we'll put all 29 electrons in. Cr = [ar] 4s1 3d5. The actual electron configuration may be rationalized in. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the. Copper Exception Electron Configuration.
From slideplayer.com
Electron configuration exceptions. There are several exceptions to the Copper Exception Electron Configuration The actual electron configuration may be rationalized in. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. Cr = [ar] 4s1 3d5. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. To understand. Copper Exception Electron Configuration.
From slideplayer.com
Basic Chemistry Chapter 5 Electronic Structure and Periodic Trends Copper Exception Electron Configuration this chemistry video tutorial covers exceptions in electron configuration. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. Cr = [ar] 4s1 3d5. Revise chromium and copper's unique. Copper Exception Electron Configuration.
From giorjqagn.blob.core.windows.net
Copper Electron Configuration Of Ion at Javier Rodriquez blog Copper Exception Electron Configuration copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. once we have the configuration for cu, the ions are simple. There are some exceptions to the order of. Copper Exception Electron Configuration.
From www.toppr.com
The electronic configuration of copper (29Cu) is. Copper Exception Electron Configuration exceptions to expected electron configurations. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. Cu = [ar] 4s1 3d10. When we write the configuration we'll put all 29 electrons in. this chemistry video tutorial covers exceptions in electron configuration. To understand why this occurs,. since. Copper Exception Electron Configuration.
From slideplayer.com
Opener 10/10 What is the relative mass and charge of an electron? ppt Copper Exception Electron Configuration Similar to chromium, this exception occurs because having a. When we write the configuration we'll put all 29 electrons in. Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. the actual electron configurations are: once we have the configuration for cu, the ions are simple. The actual electron configuration may be rationalized in.. Copper Exception Electron Configuration.
From www.youtube.com
Electron Configuration Exceptions in 4 Minutes YouTube Copper Exception Electron Configuration exceptions to expected electron configurations. The actual electron configuration may be rationalized in. To understand why this occurs,. this chemistry video tutorial covers exceptions in electron configuration. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. copper has an electron configuration of. Copper Exception Electron Configuration.
From www.youtube.com
Why Copper and Chromium are exceptions? Electronic configuration tricks Copper Exception Electron Configuration similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. this chemistry video tutorial covers exceptions in electron configuration. Similar to chromium, this exception occurs because having a. To understand why this occurs,. once we have the configuration for cu, the ions are simple. Revise chromium and copper's unique. Copper Exception Electron Configuration.
From slideplayer.com
Electrons in Atoms Electron Configuration ppt download Copper Exception Electron Configuration this chemistry video tutorial covers exceptions in electron configuration. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. the actual electron configurations are: once we have the configuration for cu, the ions are simple. To understand why this occurs,. since the core electron shells correspond to. Copper Exception Electron Configuration.
From hxeujmjoz.blob.core.windows.net
Copper Electron Configuration Orbital at Benjamin Lemus blog Copper Exception Electron Configuration copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. The actual electron configuration may be rationalized in. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. this chemistry video tutorial covers exceptions. Copper Exception Electron Configuration.
From www.pearson.com
The Electron Configurations Exceptions Pearson+ Channels Copper Exception Electron Configuration since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. Cu = [ar] 4s1 3d10. once we have the configuration for cu, the ions. Copper Exception Electron Configuration.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Exception Electron Configuration The actual electron configuration may be rationalized in. Cu = [ar] 4s1 3d10. Cr = [ar] 4s1 3d5. Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. this chemistry video tutorial covers exceptions in electron configuration. the actual electron configurations are: exceptions to expected electron configurations. When we write the configuration we'll. Copper Exception Electron Configuration.
From slideplayer.com
Electron Configuration ppt download Copper Exception Electron Configuration this chemistry video tutorial covers exceptions in electron configuration. once we have the configuration for cu, the ions are simple. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead. Copper Exception Electron Configuration.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Exception Electron Configuration Cu = [ar] 4s1 3d10. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. this chemistry video tutorial covers exceptions in electron configuration. Cr = [ar] 4s1 3d5. Revise chromium and copper's unique electron configurations as examples of exceptions to the rules,. The actual electron configuration may. Copper Exception Electron Configuration.
From www.numerade.com
SOLVED why chromium and copper have an exception for electron Copper Exception Electron Configuration this chemistry video tutorial covers exceptions in electron configuration. exceptions to expected electron configurations. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. once we have the configuration for cu, the ions are simple. When we write the configuration we'll put all. Copper Exception Electron Configuration.
From www.youtube.com
Electron Configuration Exceptions Examples Cr, Cu, Ag, and Mo YouTube Copper Exception Electron Configuration To understand why this occurs,. When we write the configuration we'll put all 29 electrons in. this chemistry video tutorial covers exceptions in electron configuration. Cr = [ar] 4s1 3d5. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. Cu = [ar] 4s1 3d10.. Copper Exception Electron Configuration.
From www.youtube.com
Electron Configuration Exceptions, Chromium and Copper Revison for A Copper Exception Electron Configuration similarly, the observed electron configuration of copper is [ar]4s 1 3d 10 instead of [ar]s 2 3d 9. Cr = [ar] 4s1 3d5. There are some exceptions to the order of filling of orbitals that is shown in figure. the actual electron configurations are: this chemistry video tutorial covers exceptions in electron configuration. When we write the. Copper Exception Electron Configuration.
From slideplayer.com
Exceptions to Predicted Electron Configurations ppt download Copper Exception Electron Configuration since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches. Similar to chromium, this exception occurs because having a. copper has an electron configuration of [ar] 3d 10 4s 1, instead of the expected [ar] 3d 9 4s 2. When we write the configuration we'll. Copper Exception Electron Configuration.