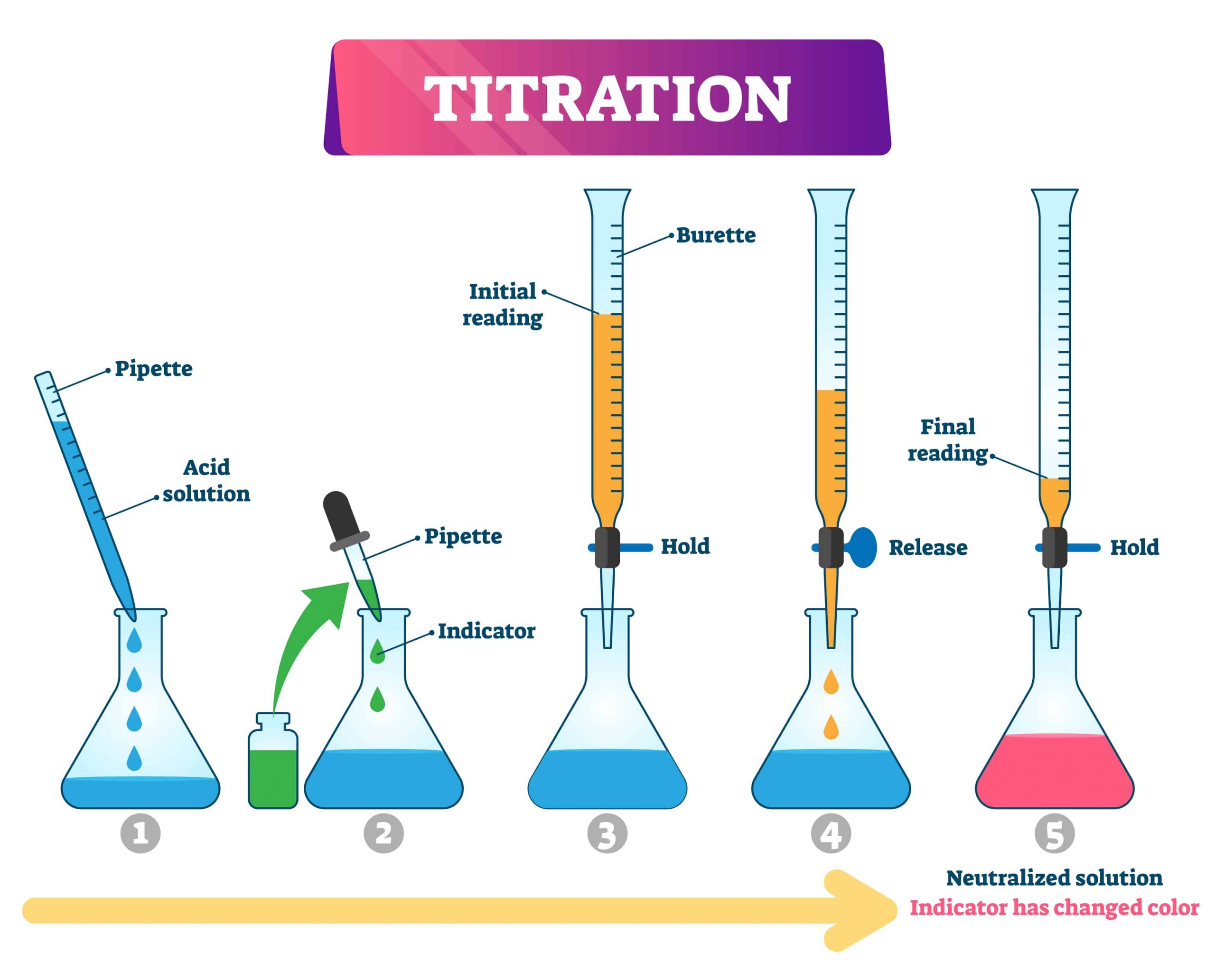
Titration Indicators Definition . Find out the characteristics, mechanism,. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. An indicator is a chemical that changes color when a titration reaches its endpoint. Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. They are usually weak acids or bases, but their. Learn how indicators work, how to choose the best one for your reaction and how to. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs.
from ar.inspiredpencil.com
Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. They are usually weak acids or bases, but their. Find out the characteristics, mechanism,. Learn how indicators work, how to choose the best one for your reaction and how to. An indicator is a chemical that changes color when a titration reaches its endpoint. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs.
Titration
Titration Indicators Definition Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. An indicator is a chemical that changes color when a titration reaches its endpoint. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn how indicators work, how to choose the best one for your reaction and how to. They are usually weak acids or bases, but their. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. Find out the characteristics, mechanism,. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Titration Indicators Definition Indicators are substances whose solutions change color due to changes in ph. An indicator is a chemical that changes color when a titration reaches its endpoint. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Learn about redox titration, a form of titrimetric analysis that involves. Titration Indicators Definition.
From www.vrogue.co
Definition Of Titration Chemistry Dictionary vrogue.co Titration Indicators Definition A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Indicators are substances whose solutions change color due to changes in ph. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. They are usually. Titration Indicators Definition.
From hxedyezjh.blob.core.windows.net
Acid Base Indicators Titration at Julie Corson blog Titration Indicators Definition They are usually weak acids or bases, but their. Learn how indicators work, how to choose the best one for your reaction and how to. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Learn about redox titration, a form of titrimetric analysis that involves. Titration Indicators Definition.
From gamesmartz.com
Titration Definition & Image GameSmartz Titration Indicators Definition An indicator is a chemical that changes color when a titration reaches its endpoint. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn how indicators work, how to choose the best one for your reaction and how to. A chemical indicator. Titration Indicators Definition.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Indicators Definition Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. They are usually weak acids or bases, but their. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Find. Titration Indicators Definition.
From 38.180.108.73
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Indicators Definition A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. They are usually weak acids or bases, but their. Learn how to measure the concentration. Titration Indicators Definition.
From dokumen.tips
(PPT) ACID BASE TITRATION INDICATORS. Titration a method of analysis Titration Indicators Definition Find out the characteristics, mechanism,. Indicators are substances whose solutions change color due to changes in ph. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Titration is a chemical process of determining the quantity of a constituent in a sample by. Titration Indicators Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Indicators Definition Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. An indicator is a chemical that changes color when a titration reaches its endpoint. A chemical. Titration Indicators Definition.
From www.slideshare.net
P h indicators Titration Indicators Definition An indicator is a chemical that changes color when a titration reaches its endpoint. Indicators are substances whose solutions change color due to changes in ph. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. Find out the characteristics, mechanism,. Learn how to measure the concentration. Titration Indicators Definition.
From www.chemistrylearner.com
Complexometric Titration Definition, Examples, and Applications Titration Indicators Definition Find out the characteristics, mechanism,. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn how indicators work, how. Titration Indicators Definition.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Indicators Definition They are usually weak acids or bases, but their. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. An indicator is a chemical that changes color when a titration reaches its endpoint. Indicators are substances whose solutions change color due to changes in ph. Titration is a chemical. Titration Indicators Definition.
From school.careers360.com
redox titration Overview, Structure, Properties & Uses Titration Indicators Definition Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. An indicator is a chemical that changes color when a titration reaches its endpoint. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. A chemical. Titration Indicators Definition.
From ar.inspiredpencil.com
Titration Setup Diagram Titration Indicators Definition Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. They are usually weak acids or bases, but their. An indicator is a chemical that changes color when a titration reaches its endpoint. Learn how to measure the concentration of a substance in a solution using titration, a technique. Titration Indicators Definition.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration Indicators Definition Find out the characteristics, mechanism,. Learn how indicators work, how to choose the best one for your reaction and how to. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves. Titration Indicators Definition.
From chemistnotes.com
Redox Titration Definition, Requirements, Indicators, and 5 Reliable Titration Indicators Definition Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Learn how to measure the concentration of a substance in a solution using. Titration Indicators Definition.
From lessonlibraryemersed.z13.web.core.windows.net
Titration Practical Questions And Answers Titration Indicators Definition Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. An indicator is a chemical that changes color when a titration reaches its endpoint. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn how. Titration Indicators Definition.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Indicators Definition They are usually weak acids or bases, but their. An indicator is a chemical that changes color when a titration reaches its endpoint. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn how indicators work, how to choose the best one. Titration Indicators Definition.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Titration Indicators Definition Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. Find out the characteristics, mechanism,. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. Titration is a technique to determine the concentration of an unknown. Titration Indicators Definition.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID9234620 Titration Indicators Definition Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. An. Titration Indicators Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Indicators Definition An indicator is a chemical that changes color when a titration reaches its endpoint. Learn how indicators work, how to choose the best one for your reaction and how to. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Find out the. Titration Indicators Definition.
From thechemistrynotes.com
Precipitation Titration Definition, Indicators, Advantages, Titration Indicators Definition Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. Learn how indicators work, how to choose the best one for your reaction and how to. Find out the characteristics, mechanism,. An indicator is a chemical that changes color when a titration reaches its endpoint. They are. Titration Indicators Definition.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Indicators Definition A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Find out the characteristics, mechanism,. Learn about redox titration, a form of titrimetric analysis that. Titration Indicators Definition.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Indicators Definition They are usually weak acids or bases, but their. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Indicators are substances whose. Titration Indicators Definition.
From slideplayer.com
Titration A pH meter or indicators are used to determine when the Titration Indicators Definition Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Find out the characteristics, mechanism,. They are usually weak acids or bases, but their. Indicators are. Titration Indicators Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Indicators Definition Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. Learn about redox titration, a form of titrimetric analysis that. Titration Indicators Definition.
From ar.inspiredpencil.com
Titration Titration Indicators Definition Find out the characteristics, mechanism,. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. Learn about redox titration, a form of titrimetric analysis that involves the reactions. Titration Indicators Definition.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Indicators Definition Indicators are substances whose solutions change color due to changes in ph. Learn how indicators work, how to choose the best one for your reaction and how to. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn about redox titration, a form of titrimetric analysis that involves. Titration Indicators Definition.
From gregorycbroman.blob.core.windows.net
Titration Definition Wiki at gregorycbroman blog Titration Indicators Definition Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. An indicator is a chemical that changes color when a titration reaches its endpoint. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an. Titration Indicators Definition.
From teachntest.org
Precipitation Titration Definition, Principle Indicators, Curve Titration Indicators Definition Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. An indicator is a chemical that changes color when a. Titration Indicators Definition.
From scienceinfo.com
Precipitation Titration Definition, Indicators, Advantages, Titration Indicators Definition Indicators are substances whose solutions change color due to changes in ph. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. An. Titration Indicators Definition.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Titration Indicators Definition An indicator is a chemical that changes color when a titration reaches its endpoint. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of. Titration Indicators Definition.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Titration Indicators Definition They are usually weak acids or bases, but their. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. A chemical indicator is a. Titration Indicators Definition.
From www.chegg.com
Solved Titration Indicators 8 5. Identify two reasons why Titration Indicators Definition Learn how to measure the concentration of a substance in a solution using titration, a technique that involves adding a reagent of known concentration to an analyte. Learn about redox titration, a form of titrimetric analysis that involves the reactions involving a change of oxidation number or transfer of electrons. An indicator is a chemical that changes color when a. Titration Indicators Definition.
From collegedunia.com
Titration Formula Types, Process & Acid Base Indicators Titration Indicators Definition Learn how indicators work, how to choose the best one for your reaction and how to. Titration is a chemical process of determining the quantity of a constituent in a sample by adding a known amount of another substance. An indicator is a chemical that changes color when a titration reaches its endpoint. Titration is a technique to determine the. Titration Indicators Definition.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titration Indicators Definition An indicator is a chemical that changes color when a titration reaches its endpoint. A chemical indicator is a substance that changes colour or turbidity in response to a chemical species, such as an acid or an alkali. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn. Titration Indicators Definition.