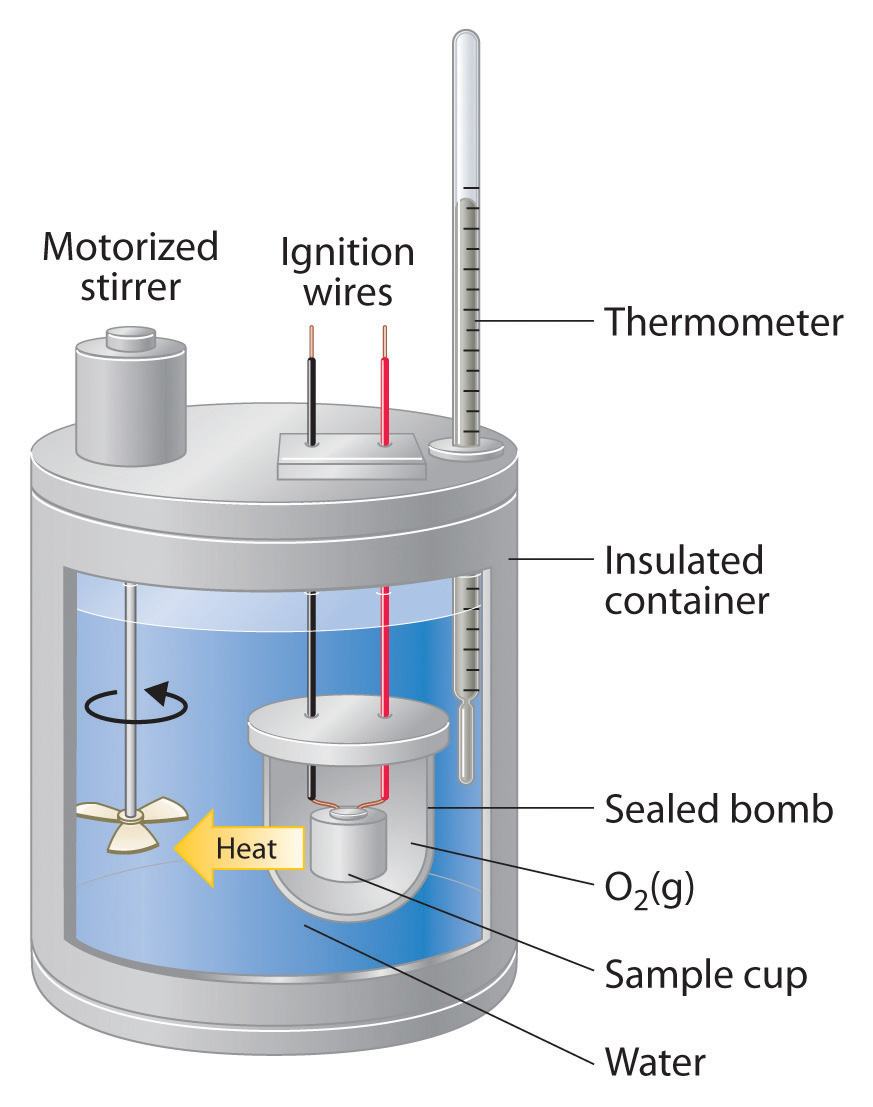
What Information Is Obtained With A Calorimeter . Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimetry is used to measure amounts of heat transferred to or from a substance.
from saylordotorg.github.io
The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity.
Calorimetry
What Information Is Obtained With A Calorimeter A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged with a calibrated object. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is.
From www.animalia-life.club
Calorimeter Diagram What Information Is Obtained With A Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry describes a set of techniques. What Information Is Obtained With A Calorimeter.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure What Information Is Obtained With A Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Technically, this is called an adiabatic surface in that no. What Information Is Obtained With A Calorimeter.
From byjus.com
What is bomb calorimeter? What Information Is Obtained With A Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. A container that prevents heat transfer in. What Information Is Obtained With A Calorimeter.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co What Information Is Obtained With A Calorimeter To do so, the heat is exchanged with a calibrated object. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the. What Information Is Obtained With A Calorimeter.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume What Information Is Obtained With A Calorimeter To do so, the heat is exchanged with a calibrated object. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A container that prevents heat transfer in or out is called a. What Information Is Obtained With A Calorimeter.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, What Information Is Obtained With A Calorimeter A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. The amount of heat released. What Information Is Obtained With A Calorimeter.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download What Information Is Obtained With A Calorimeter Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. The amount of heat. What Information Is Obtained With A Calorimeter.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet What Information Is Obtained With A Calorimeter Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry. What Information Is Obtained With A Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Information Is Obtained With A Calorimeter To do so, the heat is exchanged with a calibrated object. To do so, the heat is exchanged. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of. What Information Is Obtained With A Calorimeter.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness What Information Is Obtained With A Calorimeter The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimeters are used to measure the heat of. What Information Is Obtained With A Calorimeter.
From users.highland.edu
Calorimetry What Information Is Obtained With A Calorimeter To do so, the heat is exchanged. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A container. What Information Is Obtained With A Calorimeter.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams What Information Is Obtained With A Calorimeter Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To. What Information Is Obtained With A Calorimeter.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby What Information Is Obtained With A Calorimeter A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance.. What Information Is Obtained With A Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Information Is Obtained With A Calorimeter Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimetry is used to measure amounts of heat transferred to or from a substance. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. To. What Information Is Obtained With A Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Information Is Obtained With A Calorimeter To do so, the heat is exchanged. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimetry. What Information Is Obtained With A Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Information Is Obtained With A Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A container that prevents heat transfer in or out is called a calorimeter, and the. What Information Is Obtained With A Calorimeter.
From scienceinfo.com
Calorimeter Definition, Types and Uses What Information Is Obtained With A Calorimeter Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. To do so, the heat is exchanged. Calorimetry is the. What Information Is Obtained With A Calorimeter.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co What Information Is Obtained With A Calorimeter A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry describes a. What Information Is Obtained With A Calorimeter.
From rattibha.com
السعرات الحرارية للغذاء.. كيف بدأت وفين وصلت.. وكيف ممكن تستفيد لما What Information Is Obtained With A Calorimeter Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. A container that prevents heat transfer in or out is called a calorimeter, and the. What Information Is Obtained With A Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Information Is Obtained With A Calorimeter Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents heat transfer in or out. What Information Is Obtained With A Calorimeter.
From www.animalia-life.club
Calorimeter Diagram What Information Is Obtained With A Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimeters. What Information Is Obtained With A Calorimeter.
From www.tecquipment.com
BOMB CALORIMETER TecQuipment What Information Is Obtained With A Calorimeter The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in. What Information Is Obtained With A Calorimeter.
From slideplayer.com
A “Calorimeter”. Calorimetry Calculations When analyzing data obtained What Information Is Obtained With A Calorimeter Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic surface in that no heat. What Information Is Obtained With A Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter What Information Is Obtained With A Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimetry describes a set of techniques employed. What Information Is Obtained With A Calorimeter.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube What Information Is Obtained With A Calorimeter Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical,. What Information Is Obtained With A Calorimeter.
From byjus.com
In a calorimeter (water equivalent=40 g) are 200 g of water and 50 g of What Information Is Obtained With A Calorimeter Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. To do so, the heat is exchanged. Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. A calorimeter is an instrument that has a. What Information Is Obtained With A Calorimeter.
From ecampusontario.pressbooks.pub
3.5 Calorimétrie La Chimie Générale pour les GeeGees What Information Is Obtained With A Calorimeter The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. To do so, the heat is exchanged with a calibrated object. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. A calorimeter is an instrument that has a thermally isolated. What Information Is Obtained With A Calorimeter.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What What Information Is Obtained With A Calorimeter Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry is used to measure amounts of heat transferred to or from a substance. A. What Information Is Obtained With A Calorimeter.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer What Information Is Obtained With A Calorimeter Calorimetry is the measurement of the heat involved in a chemical reaction or physical change of state. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Technically, this is called an adiabatic surface in that no heat flows in. What Information Is Obtained With A Calorimeter.
From www.studypool.com
SOLUTION What is glass calorimeter explain with diagram and example What Information Is Obtained With A Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is the measurement of the heat involved in. What Information Is Obtained With A Calorimeter.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and What Information Is Obtained With A Calorimeter Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. To do so, the heat is exchanged with a calibrated object. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A container that prevents heat transfer in or out is called. What Information Is Obtained With A Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Information Is Obtained With A Calorimeter A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To do so, the heat is exchanged with a calibrated. What Information Is Obtained With A Calorimeter.
From saylordotorg.github.io
Calorimetry What Information Is Obtained With A Calorimeter Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical processes using devices called. To do so, the heat is exchanged. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make. What Information Is Obtained With A Calorimeter.
From manueldesnhray.blogspot.com
Identify What a Coffee Cup Calorimeter Measures. What Information Is Obtained With A Calorimeter The amount of heat released or absorbed during a reaction is determined by measuring the change in temperature of the surrounding environment. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called. What Information Is Obtained With A Calorimeter.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Information Is Obtained With A Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged with a calibrated object. Calorimetry describes a set of techniques employed to measure enthalpy changes in chemical. What Information Is Obtained With A Calorimeter.