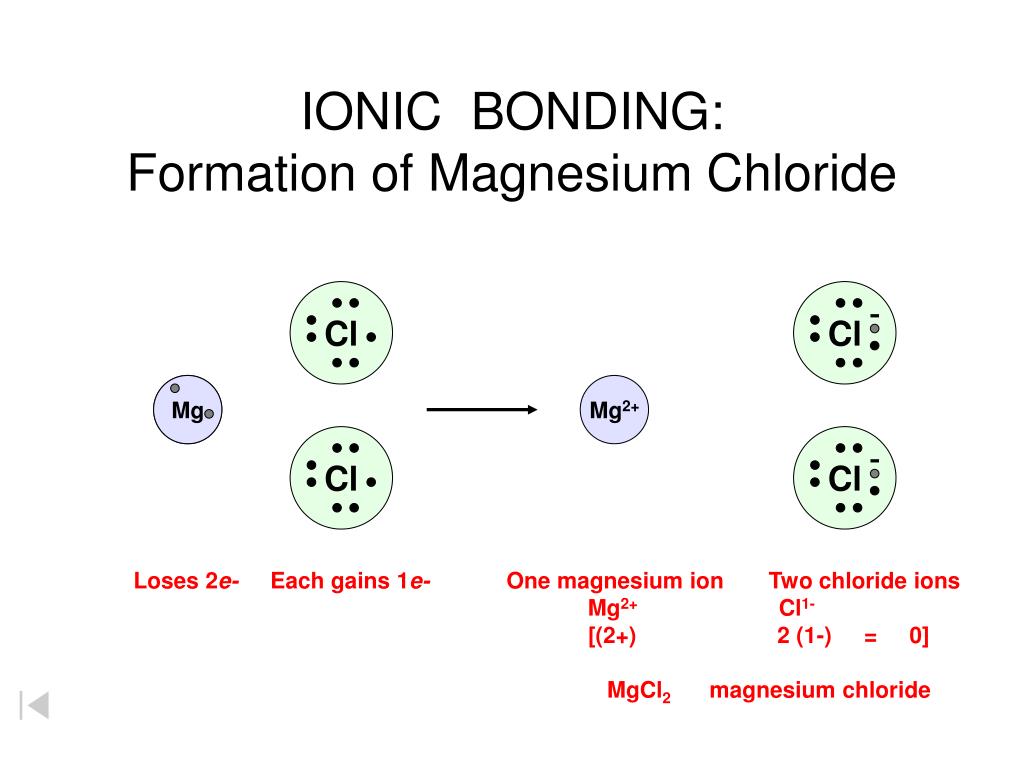
Magnesium Chloride Equation For Reaction . Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The chemical formula for magnesium chloride is mgcl2. Magnes ium + chlorine magnesium chloride. The formula for magnesium chloride is mgcl 2. Mg (s) + cl 2 (g) mg cl 2 (s) Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2].
from www.slideserve.com
Magnes ium + chlorine magnesium chloride. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Mg (s) + cl 2 (g) mg cl 2 (s) The formula for magnesium chloride is mgcl 2. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. The chemical formula for magnesium chloride is mgcl2. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous.
PPT Visualizing a Chemical Reaction PowerPoint Presentation, free
Magnesium Chloride Equation For Reaction Mg (s) + cl 2 (g) mg cl 2 (s) Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Magnes ium + chlorine magnesium chloride. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. The formula for magnesium chloride is mgcl 2. The chemical formula for magnesium chloride is mgcl2. Mg (s) + cl 2 (g) mg cl 2 (s) Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium Chloride Equation For Reaction Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnes ium + chlorine magnesium chloride. Mg (s) + cl 2 (g) mg cl 2 (s) Solid magnesium reacts with. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED Write the chemical equation for the reaction of magnesium with Magnesium Chloride Equation For Reaction The chemical formula for magnesium chloride is mgcl2. Mg (s) + cl 2 (g) mg cl 2 (s) The formula for magnesium chloride is mgcl 2. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Solid magnesium reacts with aqueous iron (iii). Magnesium Chloride Equation For Reaction.
From www.chegg.com
Solved 1 6. (25pts)Magnesium Magnesium Chloride Equation For Reaction The formula for magnesium chloride is mgcl 2. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Mg (s) + cl 2 (g) mg cl 2 (s) 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg + hcl = mgcl2 + h2. Magnesium Chloride Equation For Reaction.
From brainly.com
Solid magnesium and chlorine gas react to form solid magnesium chloride Magnesium Chloride Equation For Reaction Mg (s) + cl 2 (g) mg cl 2 (s) Magnes ium + chlorine magnesium chloride. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of. Magnesium Chloride Equation For Reaction.
From www.coursehero.com
[Solved] Magnesium chloride reacts with Sodium hydroxide. a) Write out Magnesium Chloride Equation For Reaction Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. The chemical formula for magnesium chloride is mgcl2. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Solid magnesium reacts with aqueous. Magnesium Chloride Equation For Reaction.
From tabithadesnhpotts.blogspot.com
Magnesium Chloride and Sodium Hydroxide Net Ionic Equation Magnesium Chloride Equation For Reaction Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg +. Magnesium Chloride Equation For Reaction.
From www.toppr.com
How is magnesium chloride formed by the transfer of electrons? Why does Magnesium Chloride Equation For Reaction The chemical formula for magnesium chloride is mgcl2. The formula for magnesium chloride is mgcl 2. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Mg +. Magnesium Chloride Equation For Reaction.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Magnesium Chloride Equation For Reaction Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. The formula for magnesium chloride is mgcl 2. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. 95.211 g/mol anhydrous, and 203.31. Magnesium Chloride Equation For Reaction.
From byjus.com
By the transfer of electrons, illustrate the formation of bond in Magnesium Chloride Equation For Reaction Magnes ium + chlorine magnesium chloride. The chemical formula for magnesium chloride is mgcl2. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl]. Magnesium Chloride Equation For Reaction.
From testbook.com
Magnesium Chloride Preparation, Structure, Formula, Properties Magnesium Chloride Equation For Reaction Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. The formula for magnesium chloride is mgcl 2. The chemical formula for magnesium chloride is mgcl2. Magnes ium + chlorine magnesium chloride. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction. Magnesium Chloride Equation For Reaction.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science hive Magnesium Chloride Equation For Reaction The chemical formula for magnesium chloride is mgcl2. Magnes ium + chlorine magnesium chloride. Mg (s) + cl 2 (g) mg cl 2 (s) Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Mg + hcl = mgcl2 + h2 is a. Magnesium Chloride Equation For Reaction.
From www.youtube.com
Empirical Formula of Magnesium Chloride LAB YouTube Magnesium Chloride Equation For Reaction Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Magnes ium + chlorine magnesium chloride. The formula for magnesium chloride is mgcl. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED Question 4 Magnesium chloride combines with potassium yielding Magnesium Chloride Equation For Reaction 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The formula for magnesium chloride is mgcl 2. Mg (s) + cl 2 (g) mg cl 2 (s) Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Magnes ium. Magnesium Chloride Equation For Reaction.
From www.slideserve.com
PPT Visualizing a Chemical Reaction PowerPoint Presentation, free Magnesium Chloride Equation For Reaction Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Mg (s) + cl 2 (g) mg cl 2 (s) Magnes ium + chlorine magnesium chloride. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of. Magnesium Chloride Equation For Reaction.
From klaldmnft.blob.core.windows.net
Sodium Carbonate And Magnesium Chloride Formula at Michael Knutson blog Magnesium Chloride Equation For Reaction The formula for magnesium chloride is mgcl 2. Mg (s) + cl 2 (g) mg cl 2 (s) Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. The chemical formula for magnesium chloride is mgcl2. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where. Magnesium Chloride Equation For Reaction.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium Chloride Equation For Reaction Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. The formula for magnesium chloride is mgcl 2. Mg (s) + cl 2 (g) mg. Magnesium Chloride Equation For Reaction.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Chloride Equation For Reaction Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Mg (s) + cl 2 (g) mg cl 2 (s) Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. 95.211 g/mol anhydrous,. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrogen chloride gas to form Magnesium Chloride Equation For Reaction Mg (s) + cl 2 (g) mg cl 2 (s) Magnes ium + chlorine magnesium chloride. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. The formula for magnesium chloride is mgcl 2. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg. Magnesium Chloride Equation For Reaction.
From loetsxqok.blob.core.windows.net
Atomic Number For Magnesium Chloride Formula at Scott Bowlin blog Magnesium Chloride Equation For Reaction Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. The formula for magnesium chloride is mgcl 2. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnes ium + chlorine magnesium chloride. The chemical formula for magnesium chloride is mgcl2. Mg + hcl = mgcl2. Magnesium Chloride Equation For Reaction.
From www.chegg.com
Solved 1. Use Equation 1 to write the formula for magnesium Magnesium Chloride Equation For Reaction Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. The chemical formula for magnesium chloride is mgcl2. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. 95.211 g/mol anhydrous, and 203.31. Magnesium Chloride Equation For Reaction.
From rapidelectron.blogspot.com
Give The Electron Configuration Of Magnesium And Chlorine Rapid Electron Magnesium Chloride Equation For Reaction Mg (s) + cl 2 (g) mg cl 2 (s) Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. The chemical formula for magnesium chloride is mgcl2. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine. Magnesium Chloride Equation For Reaction.
From www.youtube.com
How to Balance Mg + HCl → MgCl2 + H2 (Magnesium + Hydrochloric Acid Magnesium Chloride Equation For Reaction Mg (s) + cl 2 (g) mg cl 2 (s) Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. 95.211. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrochloric acid to produce Magnesium Chloride Equation For Reaction Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Mg (s) + cl 2 (g) mg cl 2 (s) Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. The formula for magnesium chloride is mgcl 2.. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED 1. Magnesium and hydrochloric acid react to form magnesium Magnesium Chloride Equation For Reaction Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Mg (s) + cl 2 (g) mg cl 2 (s) Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. The chemical formula for magnesium. Magnesium Chloride Equation For Reaction.
From mavink.com
Magnesium And Hcl Reaction Magnesium Chloride Equation For Reaction 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Mg (s) + cl 2 (g) mg cl 2 (s) The formula for magnesium chloride is mgcl 2. The chemical. Magnesium Chloride Equation For Reaction.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Magnesium Chloride Equation For Reaction Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Magnes ium + chlorine magnesium chloride. The chemical formula for magnesium chloride is mgcl2. Mg. Magnesium Chloride Equation For Reaction.
From www.youtube.com
Type of Reaction for Mg + HCl = MgCl2 + H2 YouTube Magnesium Chloride Equation For Reaction Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg (s) + cl 2 (g) mg cl 2 (s) Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron. Magnesium Chloride Equation For Reaction.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Chloride Equation For Reaction Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mg + cl = mgcl2. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED Magnesium reacts with hydrochloric acid, HCl, to form magnesium Magnesium Chloride Equation For Reaction 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The formula for magnesium chloride is mgcl 2. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED (a) Write the balanced chemical equation following reaction. 1 Magnesium Chloride Equation For Reaction 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. The formula for magnesium chloride is mgcl 2. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Magnes ium + chlorine magnesium chloride. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid. Magnesium Chloride Equation For Reaction.
From www.youtube.com
How to draw dot and cross diagram of magnesium chloride ionic compound Magnesium Chloride Equation For Reaction Magnes ium + chlorine magnesium chloride. The formula for magnesium chloride is mgcl 2. Mg (s) + cl 2 (g) mg cl 2 (s) Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Mgcl2 + naoh = mg(oh)2 + nacl is a. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Magnesium Chloride Equation For Reaction Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. The chemical formula for magnesium chloride is mgcl2. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Magnes ium + chlorine magnesium chloride. Solid magnesium reacts with aqueous iron (iii) chloride to produce solid. Magnesium Chloride Equation For Reaction.
From koltenfersrubio.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Chloride Equation For Reaction Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. The chemical formula for magnesium chloride is mgcl2. Mg (s) + cl 2 (g) mg cl 2 (s) 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate, is the molar mass of the. Mgcl2 + naoh = mg(oh)2 + nacl is a. Magnesium Chloride Equation For Reaction.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrogen chloride gas to form Magnesium Chloride Equation For Reaction Solid magnesium reacts with aqueous iron (iii) chloride to produce solid iron and aqueous magnesium chloride write a balanced chemical. Mg + cl = mgcl2 is a synthesis reaction where one mole of magnesium [mg] and two moles of chlorine [cl] combine to form one mole of. Magnes ium + chlorine magnesium chloride. 95.211 g/mol anhydrous, and 203.31 g/mol hexahydrate,. Magnesium Chloride Equation For Reaction.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Magnesium Chloride Equation For Reaction The formula for magnesium chloride is mgcl 2. Mgcl2 + naoh = mg(oh)2 + nacl is a double displacement (metathesis) reaction where one mole of aqueous magnesium chloride [mgcl 2]. Mg + hcl = mgcl2 + h2 is a single displacement (substitution) reaction where one mole of solid magnesium [mg] and two moles of aqueous. Solid magnesium reacts with aqueous. Magnesium Chloride Equation For Reaction.