Calorimetry And Specific Heat Lab Answers . Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. What is the procedure for heating a metal to an exact, but measured, temperature? Specific heat capacity can be described as a substance's resistance to temperature changes. 10 to 30g metal should be. Purpose explore how the specific heat of a substance can be determined. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Which substance has a greater specific heat.
from www.studocu.com
What is the procedure for heating a metal to an exact, but measured, temperature? Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. Which substance has a greater specific heat. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Purpose explore how the specific heat of a substance can be determined. Specific heat capacity can be described as a substance's resistance to temperature changes. 10 to 30g metal should be. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc?
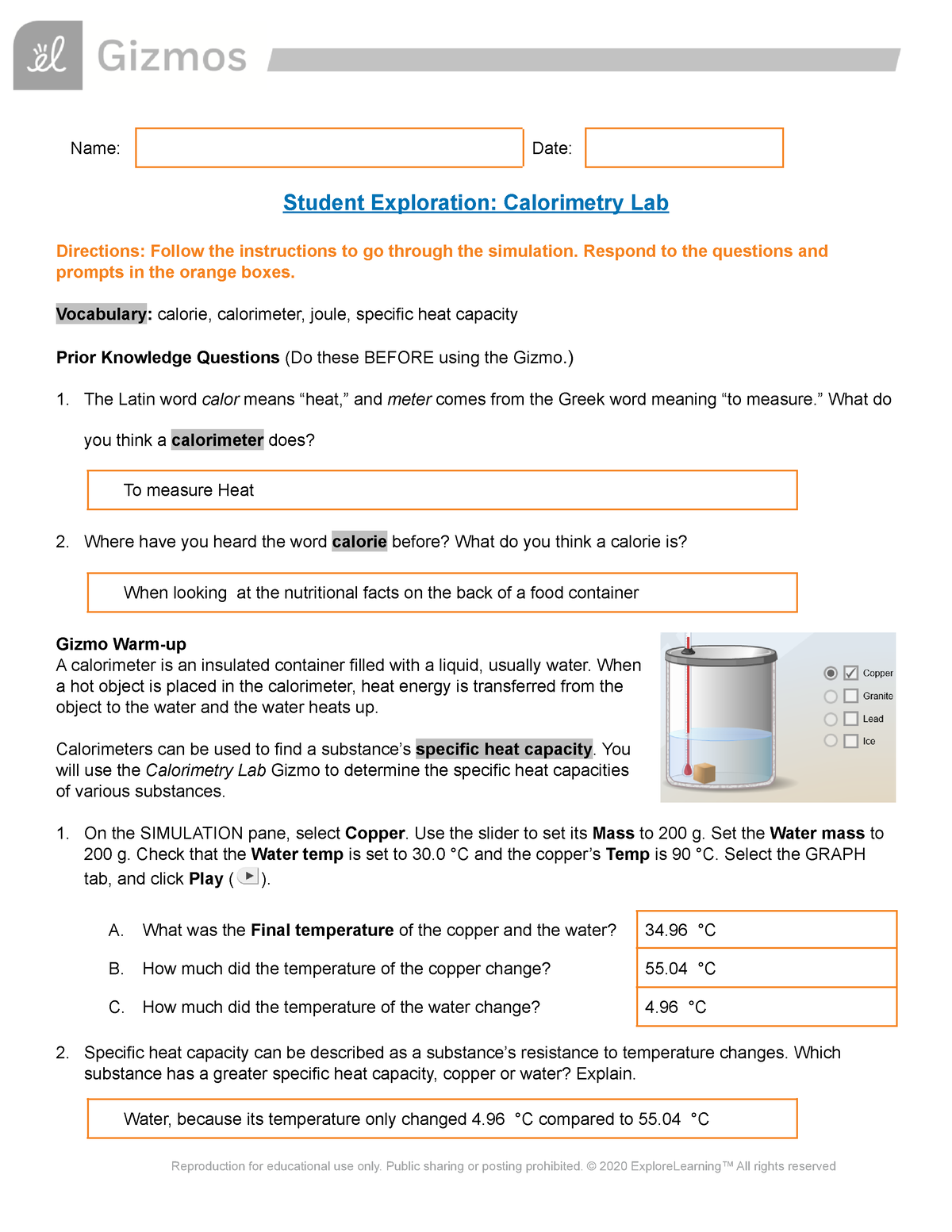
Student Exploration Calorimetry Lab Name Date Student Exploration
Calorimetry And Specific Heat Lab Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. Specific heat capacity can be described as a substance's resistance to temperature changes. Which substance has a greater specific heat. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 10 to 30g metal should be. What is the procedure for heating a metal to an exact, but measured, temperature? Purpose explore how the specific heat of a substance can be determined. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in.
From www.studocu.com
Calorimetry and Specific Heat Lab Question Using a calorimeter, how Calorimetry And Specific Heat Lab Answers What is the procedure for heating a metal to an exact, but measured, temperature? How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. The amount of heat absorbed or released (q). Calorimetry And Specific Heat Lab Answers.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Calorimetry And Specific Heat Lab Answers Purpose explore how the specific heat of a substance can be determined. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. The amount of heat absorbed or released (q) by the. Calorimetry And Specific Heat Lab Answers.
From www.studocu.com
Experiment 25 calorimetry Bryce Augustine Chem 005 Experiment 25 Calorimetry And Specific Heat Lab Answers Specific heat capacity can be described as a substance's resistance to temperature changes. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Purpose explore how the specific heat of a substance can be determined. Which substance has a greater specific heat. 10 to 30g metal should be. Study with quizlet and memorize flashcards. Calorimetry And Specific Heat Lab Answers.
From www.studocu.com
Calorimetry Experiment 25 Calorimetry To determine the specific heat Calorimetry And Specific Heat Lab Answers Purpose explore how the specific heat of a substance can be determined. Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. Specific heat capacity can be described as a substance's resistance to temperature changes. Which substance has a greater specific heat. What is the procedure for heating. Calorimetry And Specific Heat Lab Answers.
From www.studocu.com
32100704studentguide Copyright © Edgenuity Inc. Lab Calorimetry Calorimetry And Specific Heat Lab Answers Specific heat capacity can be described as a substance's resistance to temperature changes. Purpose explore how the specific heat of a substance can be determined. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Which substance has a greater specific heat. Study with quizlet and memorize flashcards containing terms like beryllium is a. Calorimetry And Specific Heat Lab Answers.
From www.scribd.com
Lab 07Specific Heat & Calorimetry.pdf Heat Temperature Calorimetry And Specific Heat Lab Answers Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. What is the procedure for heating a metal to an exact, but measured, temperature? Purpose explore how the specific heat of a substance can be determined. 10 to 30g metal should be. How much energy is needed to. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Solved REPORT FOR EXPERIMENT5 Calorimetry and Specific Heat Calorimetry And Specific Heat Lab Answers Purpose explore how the specific heat of a substance can be determined. Which substance has a greater specific heat. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. Study with quizlet. Calorimetry And Specific Heat Lab Answers.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Calorimetry And Specific Heat Lab Answers Specific heat capacity can be described as a substance's resistance to temperature changes. Which substance has a greater specific heat. Purpose explore how the specific heat of a substance can be determined. Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. How much energy is needed to. Calorimetry And Specific Heat Lab Answers.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimetry And Specific Heat Lab Answers Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 10 to 30g metal should be. The amount of heat absorbed or released (q) by the object depends on its mass (m),. Calorimetry And Specific Heat Lab Answers.
From worksheet.iemays.edu.pe
Specific Heat And Calorimetry Worksheet Calorimetry And Specific Heat Lab Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Which substance has a greater specific heat. What is the procedure for heating a metal to an exact, but measured, temperature? Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. The. Calorimetry And Specific Heat Lab Answers.
From studylibfreytag.z21.web.core.windows.net
Calorimetry Problems Worksheet Answers Calorimetry And Specific Heat Lab Answers Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. What is the procedure for heating a metal to an exact, but measured, temperature? Purpose explore how the specific heat of a substance can be determined. 10 to 30g metal should be. How much energy is needed to. Calorimetry And Specific Heat Lab Answers.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool Calorimetry And Specific Heat Lab Answers Purpose explore how the specific heat of a substance can be determined. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Which substance has a greater specific heat. What. Calorimetry And Specific Heat Lab Answers.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Calorimetry And Specific Heat Lab Answers Which substance has a greater specific heat. What is the procedure for heating a metal to an exact, but measured, temperature? Purpose explore how the specific heat of a substance can be determined. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. 10 to 30g metal should. Calorimetry And Specific Heat Lab Answers.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry And Specific Heat Lab Answers Specific heat capacity can be described as a substance's resistance to temperature changes. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the procedure for heating a metal to an exact, but measured, temperature? 10 to 30g metal should be. Study with quizlet and memorize flashcards containing terms like beryllium is. Calorimetry And Specific Heat Lab Answers.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Calorimetry And Specific Heat Lab Answers The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Purpose explore how the specific heat of a substance can be determined. Specific heat capacity can be described as a substance's resistance to temperature changes. What is the procedure for heating a metal to an exact,. Calorimetry And Specific Heat Lab Answers.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry And Specific Heat Lab Answers Purpose explore how the specific heat of a substance can be determined. Specific heat capacity can be described as a substance's resistance to temperature changes. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Solved Calorimetry Heat of Fusion and Specific Heat(we are Calorimetry And Specific Heat Lab Answers Which substance has a greater specific heat. Specific heat capacity can be described as a substance's resistance to temperature changes. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Purpose explore how the specific heat of a substance can be determined. Assuming the specific heat. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Lab 3. Calorimetry Measurement of Specific Heat Calorimetry And Specific Heat Lab Answers Which substance has a greater specific heat. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. What is the procedure for heating a. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Solved LAB REPORT CALORIMETRY Table 11.1 Specific heat. Calorimetry And Specific Heat Lab Answers 10 to 30g metal should be. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Which substance has a greater specific heat. Specific heat capacity can be described as a substance's resistance to temperature changes. Study with quizlet and memorize flashcards containing terms like beryllium. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Solved connect Calorimetry TRY. HEAT Calorimetry And Specific Heat Lab Answers The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 10 to 30g metal should be. What is the procedure for heating a metal to an exact, but measured, temperature?. Calorimetry And Specific Heat Lab Answers.
From www.studypool.com
SOLUTION Gizmo student exploration calorimetry lab answer key Studypool Calorimetry And Specific Heat Lab Answers The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Purpose explore how the specific heat of a substance can be determined. Which substance has a greater specific heat. What. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Laboratory 9 CALORIMETRY SPECIFIC HEAT OF A METAL Calorimetry And Specific Heat Lab Answers Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Which substance has a greater specific heat. What is the procedure for heating a. Calorimetry And Specific Heat Lab Answers.
From undangan-undangan-undangan.blogspot.com
Student Exploration Calorimetry Lab Gizmo Answers 35+ Pages Answer in Calorimetry And Specific Heat Lab Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Purpose explore how the specific heat of a substance can be determined. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. The amount of heat absorbed or released (q) by the. Calorimetry And Specific Heat Lab Answers.
From brainly.com
please i need it Lab Calorimetry and Specific Heat Calorimetry And Specific Heat Lab Answers What is the procedure for heating a metal to an exact, but measured, temperature? Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. 10 to 30g metal should be. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat. Calorimetry And Specific Heat Lab Answers.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry And Specific Heat Lab Answers Purpose explore how the specific heat of a substance can be determined. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Which substance has a greater specific heat. 10 to 30g metal should be. Specific heat capacity can be described as a substance's resistance to temperature changes.. Calorimetry And Specific Heat Lab Answers.
From kimora-kbowen.blogspot.com
Temperature and Specific Heat Lab 4 Answers Calorimetry And Specific Heat Lab Answers Which substance has a greater specific heat. Specific heat capacity can be described as a substance's resistance to temperature changes. 10 to 30g metal should be. Purpose explore how the specific heat of a substance can be determined. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Study with quizlet and memorize flashcards. Calorimetry And Specific Heat Lab Answers.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry And Specific Heat Lab Answers The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. 10 to 30g metal should be. Purpose explore how the specific heat of a substance can be determined. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Study with. Calorimetry And Specific Heat Lab Answers.
From www.studocu.com
Copy of Lab 5 Calorimetry and Specific Heat Lab Calorimetry and Calorimetry And Specific Heat Lab Answers The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong,. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Laboratory 9 CALORIMETRY SPECIFIC HEAT OF A METAL Calorimetry And Specific Heat Lab Answers 10 to 30g metal should be. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. Specific heat capacity can be described as a substance's resistance to temperature changes. Which substance has. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Solved Calorimetry Lab Report o PART A. Specific Heat Calorimetry And Specific Heat Lab Answers 10 to 30g metal should be. What is the procedure for heating a metal to an exact, but measured, temperature? Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. Which substance has a greater specific heat. Specific heat capacity can be described as a substance's resistance to. Calorimetry And Specific Heat Lab Answers.
From www.studocu.com
Student Exploration Calorimetry Lab Name Date Student Exploration Calorimetry And Specific Heat Lab Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the procedure for heating a metal to an exact, but measured, temperature? The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. 10 to 30g metal should be.. Calorimetry And Specific Heat Lab Answers.
From www.chegg.com
Solved Data Table for the Specific Heat and Calorimeter Lab Calorimetry And Specific Heat Lab Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Purpose explore how the specific heat of a substance can be determined. What is the procedure for heating a metal to an exact, but measured, temperature? The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat. Calorimetry And Specific Heat Lab Answers.
From studylib.net
Calorimetry Worksheet Calorimetry And Specific Heat Lab Answers The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. 10 to 30g metal should be. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. How much energy is needed to change the. Calorimetry And Specific Heat Lab Answers.
From www.studocu.com
LABManual Expt 9 notes and answers for Lab 3 notes from Dr ross s Calorimetry And Specific Heat Lab Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. 10 to 30g metal should be. The amount of heat absorbed or released (q) by the object depends on its mass (m),. Calorimetry And Specific Heat Lab Answers.
From lessonfullbaum.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheet Calorimetry And Specific Heat Lab Answers Assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat in joules produced. Specific heat capacity can be described as a substance's resistance to temperature changes. What is the procedure for heating a metal to an exact, but measured, temperature? 10 to 30g metal should be. How much energy is needed. Calorimetry And Specific Heat Lab Answers.