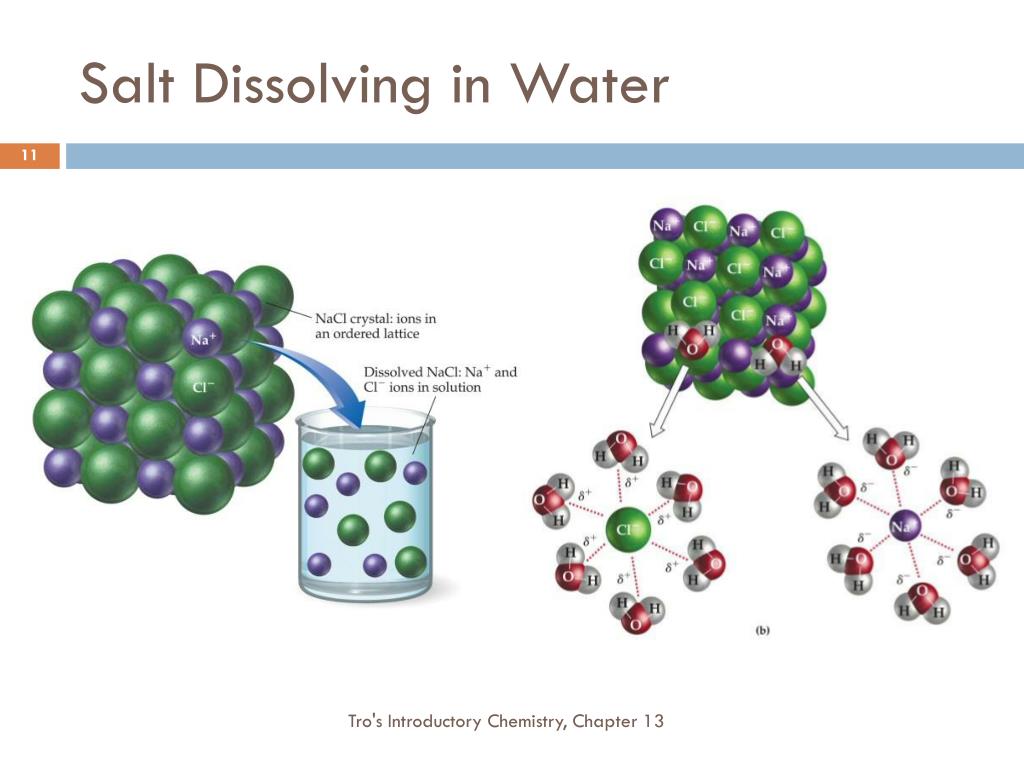
Does Salt Dissolve Liquids . Basically, polar (magnetically charged) solids. The new world encyclopedia explains that like dissolves into like; Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. We will first examine the process that occurs when. Water typically dissolves many ionic compounds and polar molecules. The water molecules surround the ions and pull them. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Does it dissolve in the water? Some salts are actually insoluble even in water. Nonpolar molecules such as those found in grease or oil do not dissolve in. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. What happens to the salt? Put on the lid and shake the cup for about 20 to 30 seconds. What does the mixture look like? When salt is added to water, the positive and negative ions separate.
from www.slideserve.com
What does the mixture look like? Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Some salts are actually insoluble even in water. Basically, polar (magnetically charged) solids. We will first examine the process that occurs when. The water molecules surround the ions and pull them. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. What happens to the salt? Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Nonpolar molecules such as those found in grease or oil do not dissolve in.
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID
Does Salt Dissolve Liquids Basically, polar (magnetically charged) solids. Some salts are actually insoluble even in water. When salt is added to water, the positive and negative ions separate. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. What happens to the salt? What does the mixture look like? Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Basically, polar (magnetically charged) solids. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. The water molecules surround the ions and pull them. We will first examine the process that occurs when. The new world encyclopedia explains that like dissolves into like; Nonpolar molecules such as those found in grease or oil do not dissolve in. Water typically dissolves many ionic compounds and polar molecules. Put on the lid and shake the cup for about 20 to 30 seconds. Does it dissolve in the water?
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow Does Salt Dissolve Liquids Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Basically, polar (magnetically charged) solids. Nonpolar molecules such as those found in grease or oil do not dissolve in. What does the mixture look like? What happens to the salt? Nonpolar molecules, such as those found in grease or oil, do not. Does Salt Dissolve Liquids.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Does Salt Dissolve Liquids We will first examine the process that occurs when. The water molecules surround the ions and pull them. Water typically dissolves many ionic compounds and polar molecules. Put on the lid and shake the cup for about 20 to 30 seconds. What happens to the salt? Does it dissolve in the water? Basically, polar (magnetically charged) solids. What does the. Does Salt Dissolve Liquids.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Does Salt Dissolve Liquids Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. What does the mixture look like? We will first examine the process that occurs when. Water typically dissolves many ionic compounds and polar molecules. Does it dissolve in the water? Basically, polar (magnetically charged) solids. The new world encyclopedia explains that like dissolves into like;. Does Salt Dissolve Liquids.
From www.youtube.com
table salt dissolves in water YouTube Does Salt Dissolve Liquids Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. What does the mixture look like? The new world encyclopedia explains that like dissolves into like; Salt is an ionic compound, meaning it is a substance made up of. Does Salt Dissolve Liquids.
From energyeducation.ca
Solute Energy Education Does Salt Dissolve Liquids What happens to the salt? Basically, polar (magnetically charged) solids. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. Nonpolar molecules such as. Does Salt Dissolve Liquids.
From www.wikidoc.org
Solution wikidoc Does Salt Dissolve Liquids Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Put on the lid and shake the cup for about 20 to 30 seconds. The new world encyclopedia explains that like dissolves into like; Does it dissolve in the water? We will first examine the process that occurs when. Salt is an ionic compound, meaning. Does Salt Dissolve Liquids.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube Does Salt Dissolve Liquids Does it dissolve in the water? Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. When salt is added to water, the positive and negative ions separate. Nonpolar molecules such as those found in grease or oil do. Does Salt Dissolve Liquids.
From www.ck12.org
Solubility CK12 Foundation Does Salt Dissolve Liquids Does it dissolve in the water? Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. Put on the lid and shake the cup for about 20 to 30 seconds. What does the mixture look like? The water molecules. Does Salt Dissolve Liquids.
From www.twinkl.kr
What is Dissolving? Answered Twinkl Teaching Wiki Does Salt Dissolve Liquids When salt is added to water, the positive and negative ions separate. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Some salts are actually insoluble even in water. We will first examine the process that occurs when. Water typically dissolves many ionic compounds and polar molecules. What does the mixture. Does Salt Dissolve Liquids.
From slideplayer.com
Water. ppt download Does Salt Dissolve Liquids What does the mixture look like? Some salts are actually insoluble even in water. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. Put on the lid and shake the cup for about 20 to 30 seconds. Water. Does Salt Dissolve Liquids.
From www.youtube.com
Essential Chemistry Dissolving Salt in Water YouTube Does Salt Dissolve Liquids What does the mixture look like? The water molecules surround the ions and pull them. Put on the lid and shake the cup for about 20 to 30 seconds. Some salts are actually insoluble even in water. Water typically dissolves many ionic compounds and polar molecules. The new world encyclopedia explains that like dissolves into like; Basically, polar (magnetically charged). Does Salt Dissolve Liquids.
From www.slideserve.com
PPT Introductory Chemistry , 3 rd Edition Nivaldo Tro PowerPoint Does Salt Dissolve Liquids Some salts are actually insoluble even in water. We will first examine the process that occurs when. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. Water typically dissolves many ionic compounds and polar molecules. What happens to. Does Salt Dissolve Liquids.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Does Salt Dissolve Liquids Put on the lid and shake the cup for about 20 to 30 seconds. The new world encyclopedia explains that like dissolves into like; Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. What happens to the salt? Does it dissolve in the water? The water molecules surround the ions and pull them. Salt. Does Salt Dissolve Liquids.
From www.shalom-education.com
Ionic Compounds Edexcel GCSE Chemistry Revision Does Salt Dissolve Liquids The new world encyclopedia explains that like dissolves into like; Nonpolar molecules such as those found in grease or oil do not dissolve in. What does the mixture look like? Put on the lid and shake the cup for about 20 to 30 seconds. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt. Does Salt Dissolve Liquids.
From www.alamy.com
Saline solution salt liquid water Stock Vector Images Alamy Does Salt Dissolve Liquids Put on the lid and shake the cup for about 20 to 30 seconds. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them. We will first examine the process that occurs when. What happens to the salt? Basically, polar (magnetically charged) solids. Nonpolar molecules, such as those found. Does Salt Dissolve Liquids.
From www.pinterest.com
Chemistry for Kids Making and Separating Mixtures Chemistry for Does Salt Dissolve Liquids Some salts are actually insoluble even in water. The new world encyclopedia explains that like dissolves into like; When salt is added to water, the positive and negative ions separate. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Put on the lid and shake the cup for about 20 to. Does Salt Dissolve Liquids.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Does Salt Dissolve Liquids What happens to the salt? Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. When salt is added to water, the positive and negative ions separate. The new world encyclopedia explains that like dissolves into like; Basically, polar (magnetically charged) solids. The water molecules surround the ions and pull them. Water typically dissolves many. Does Salt Dissolve Liquids.
From mungfali.com
Dissolving Salt In Water Diagram Does Salt Dissolve Liquids We will first examine the process that occurs when. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. The water molecules surround the ions and pull them. Does it dissolve in the water? What does the mixture look. Does Salt Dissolve Liquids.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Does Salt Dissolve Liquids What does the mixture look like? Basically, polar (magnetically charged) solids. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Nonpolar molecules such as those found in grease or oil do not dissolve in. When salt is added to water, the positive and negative ions separate. The new world encyclopedia explains. Does Salt Dissolve Liquids.
From www.slideshare.net
How salt dissolves Does Salt Dissolve Liquids What does the mixture look like? The new world encyclopedia explains that like dissolves into like; Basically, polar (magnetically charged) solids. Put on the lid and shake the cup for about 20 to 30 seconds. The water molecules surround the ions and pull them. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules, such as those found in. Does Salt Dissolve Liquids.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Does Salt Dissolve Liquids Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. The new world encyclopedia explains that like dissolves into like; The water molecules surround the ions and pull them. When salt is added to water, the positive and negative. Does Salt Dissolve Liquids.
From loeiulzbj.blob.core.windows.net
Why The Salt Dissolves In Water at Beverly Leach blog Does Salt Dissolve Liquids Does it dissolve in the water? Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Nonpolar molecules such as those found in grease or oil do not dissolve in. What does the mixture look like? What happens to the salt? Put on the lid and shake the cup for about 20 to 30 seconds.. Does Salt Dissolve Liquids.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Does Salt Dissolve Liquids What does the mixture look like? Some salts are actually insoluble even in water. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Basically, polar (magnetically charged) solids. Does it dissolve in the water? Put on the lid and shake the cup for about 20 to 30 seconds. The new world. Does Salt Dissolve Liquids.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Does Salt Dissolve Liquids Some salts are actually insoluble even in water. Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. The water molecules surround the ions and pull them. Nonpolar molecules such as those found in grease or oil do not dissolve in. Water typically dissolves many ionic compounds and polar molecules. We will first examine the. Does Salt Dissolve Liquids.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Does Salt Dissolve Liquids Water typically dissolves many ionic compounds and polar molecules. Does it dissolve in the water? The water molecules surround the ions and pull them. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Some salts are actually insoluble even in water. When salt is added to water, the positive and negative. Does Salt Dissolve Liquids.
From www.youtube.com
Does salt dissolve in water? YouTube Does Salt Dissolve Liquids What does the mixture look like? Nonpolar molecules such as those found in grease or oil do not dissolve in. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Basically, polar (magnetically charged) solids. We will first examine the process that occurs when. The new world encyclopedia explains that like dissolves. Does Salt Dissolve Liquids.
From wirewiringlorraine.z13.web.core.windows.net
Diagram Of Salt Dissolved In Water Does Salt Dissolve Liquids What happens to the salt? The new world encyclopedia explains that like dissolves into like; Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. We will first examine the process that occurs when. Put on the. Does Salt Dissolve Liquids.
From exonfafiq.blob.core.windows.net
Does Salt Dissolve In Room Temperature Water at Peter Allen blog Does Salt Dissolve Liquids Water typically dissolves many ionic compounds and polar molecules. The new world encyclopedia explains that like dissolves into like; Some salts are actually insoluble even in water. Does it dissolve in the water? Basically, polar (magnetically charged) solids. Nonpolar molecules such as those found in grease or oil do not dissolve in. Nonpolar molecules, such as those found in grease. Does Salt Dissolve Liquids.
From loeikvgrb.blob.core.windows.net
How Long Does It Take For Rock Salt To Dissolve at Michael Kato blog Does Salt Dissolve Liquids When salt is added to water, the positive and negative ions separate. Some salts are actually insoluble even in water. Does it dissolve in the water? Water typically dissolves many ionic compounds and polar molecules. Put on the lid and shake the cup for about 20 to 30 seconds. The water molecules surround the ions and pull them. We will. Does Salt Dissolve Liquids.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Does Salt Dissolve Liquids What does the mixture look like? The water molecules surround the ions and pull them. Some salts are actually insoluble even in water. Solids dissolving in a liquid solvent (usually water in chemistry experiments) create a solution, and salt dissolving in water is a classic example of how a polar solute behaves in a. The new world encyclopedia explains that. Does Salt Dissolve Liquids.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Does Salt Dissolve Liquids Some salts are actually insoluble even in water. The water molecules surround the ions and pull them. Basically, polar (magnetically charged) solids. The new world encyclopedia explains that like dissolves into like; Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. What happens to the salt? Does it dissolve in the water? Water typically. Does Salt Dissolve Liquids.
From www.youtube.com
Alevel biology why does salt dissolve in water? YouTube Does Salt Dissolve Liquids Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Some salts are actually insoluble even in water. Put on the lid and shake the cup for about 20 to 30 seconds. Water typically dissolves many ionic compounds and polar molecules. Does it dissolve in the water? Basically, polar (magnetically charged) solids.. Does Salt Dissolve Liquids.
From www.youtube.com
How Salt Dissolves in Water? YouTube Does Salt Dissolve Liquids When salt is added to water, the positive and negative ions separate. Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. Does it dissolve in the water? Basically, polar (magnetically charged) solids. The water molecules surround the ions and pull them. Some salts are actually insoluble even in water. Solids dissolving. Does Salt Dissolve Liquids.
From schematicfixtrysted.z22.web.core.windows.net
Diagram Of Salt Dissolving In Water Does Salt Dissolve Liquids What happens to the salt? Water typically dissolves many ionic compounds and polar molecules. Some salts are actually insoluble even in water. Nonpolar molecules such as those found in grease or oil do not dissolve in. Does it dissolve in the water? Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions.. Does Salt Dissolve Liquids.
From webmis.highland.cc.il.us
Aqueous Solutions Does Salt Dissolve Liquids Nonpolar molecules, such as those found in grease or oil, do not dissolve in water. We will first examine the process that occurs when. What happens to the salt? The new world encyclopedia explains that like dissolves into like; Salt is an ionic compound, meaning it is a substance made up of electrically charged molecules called ions. When salt is. Does Salt Dissolve Liquids.